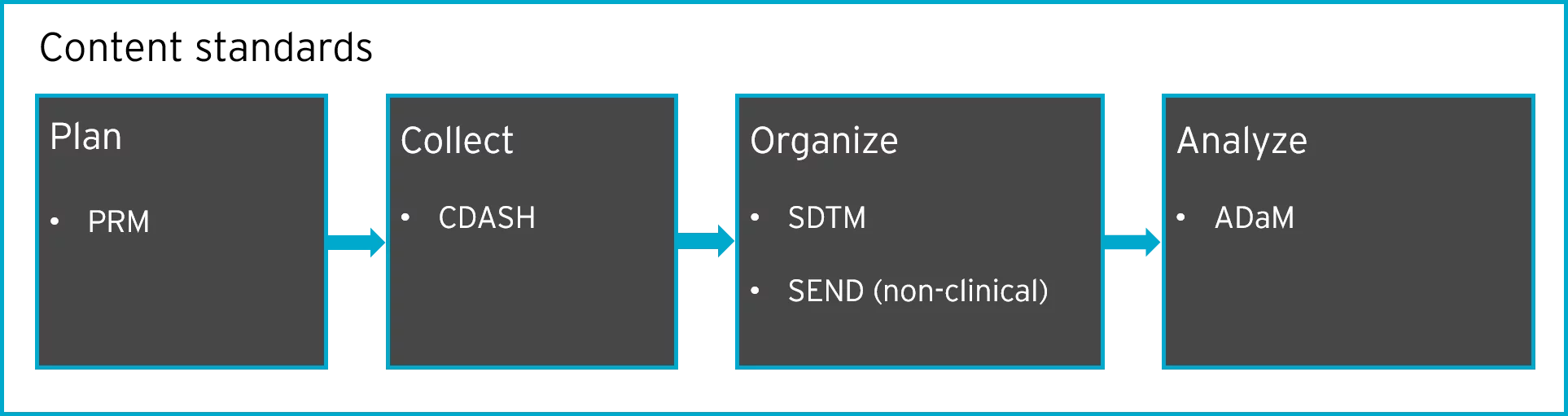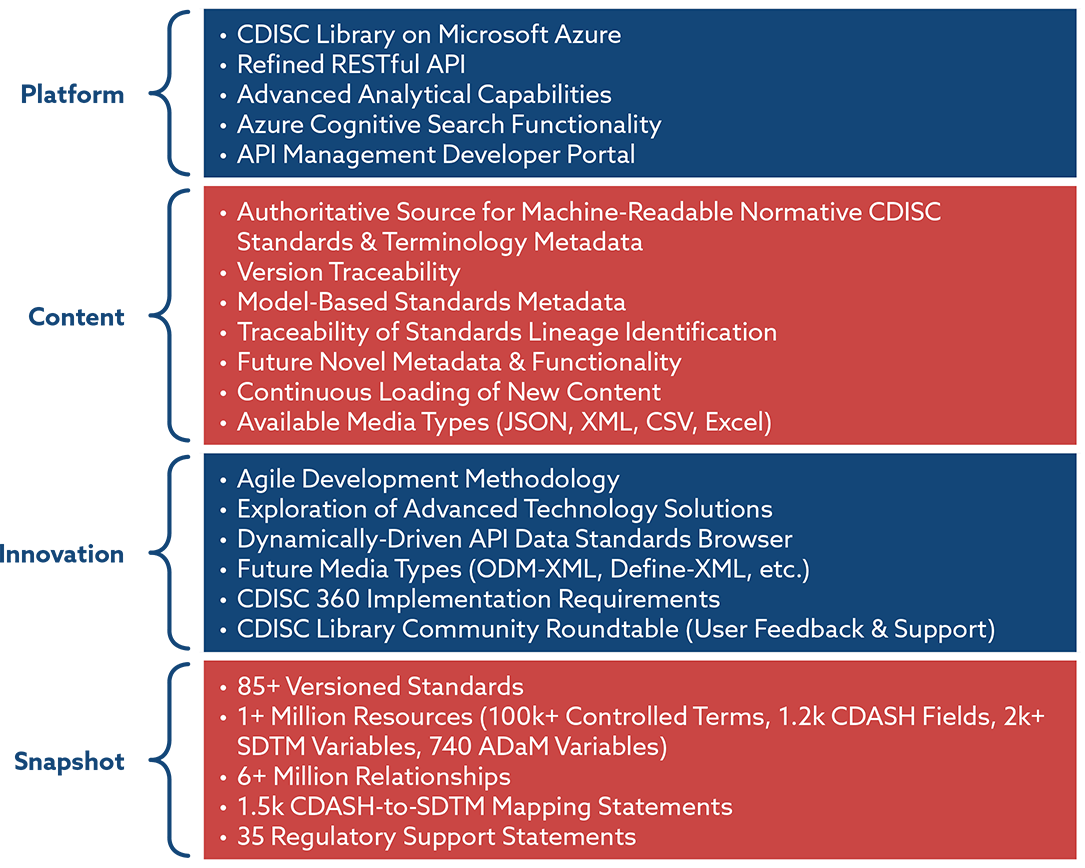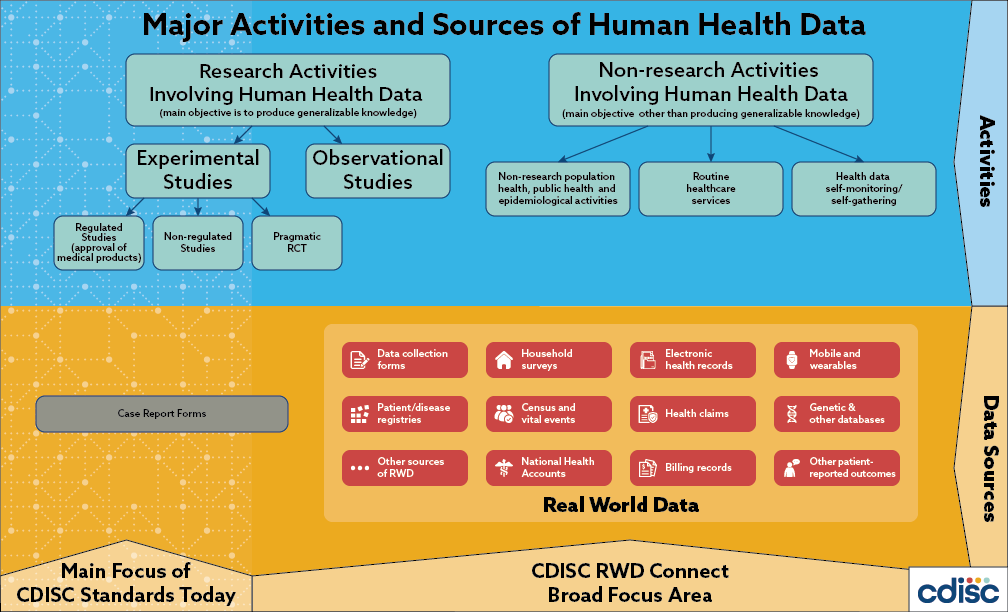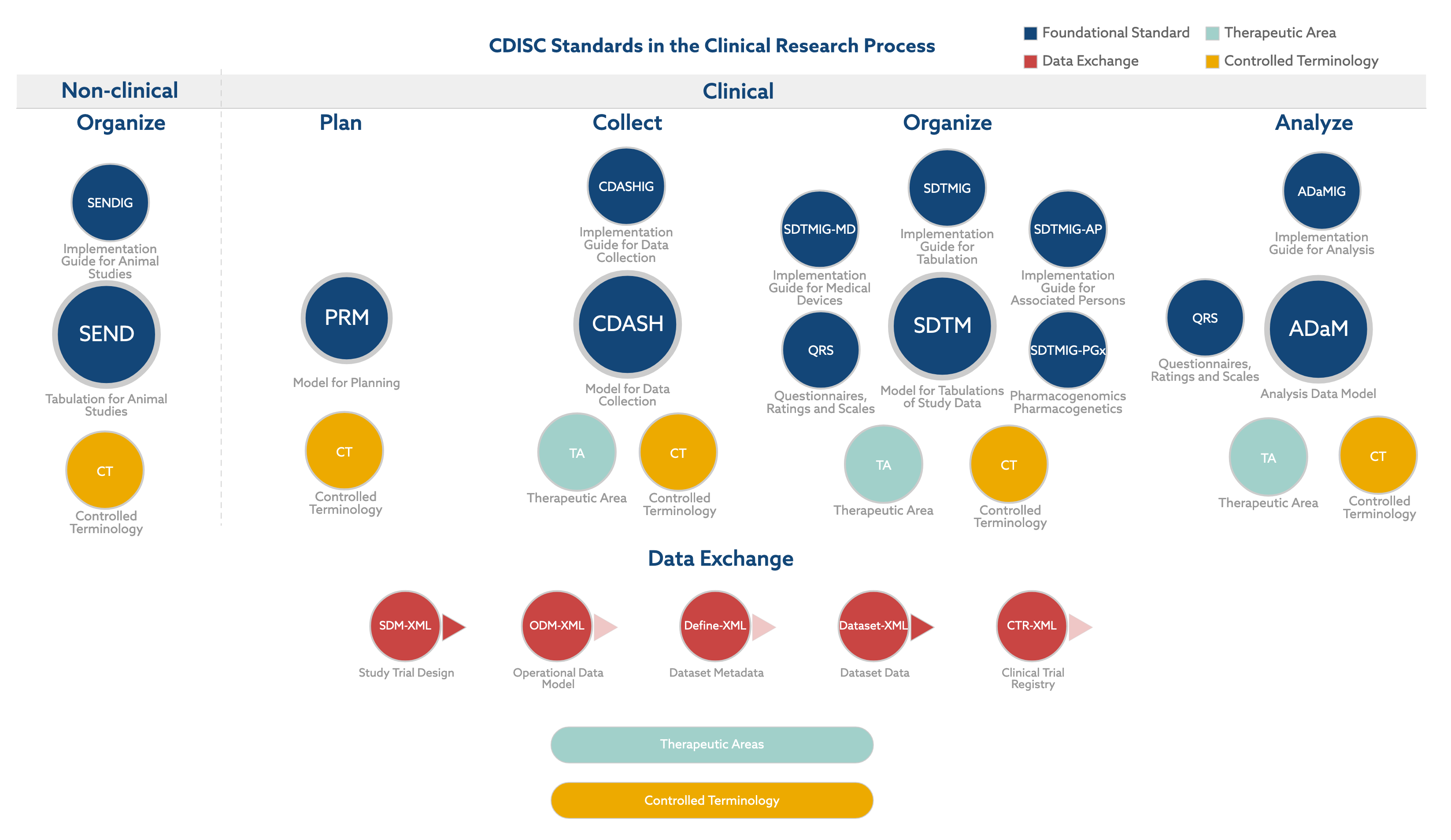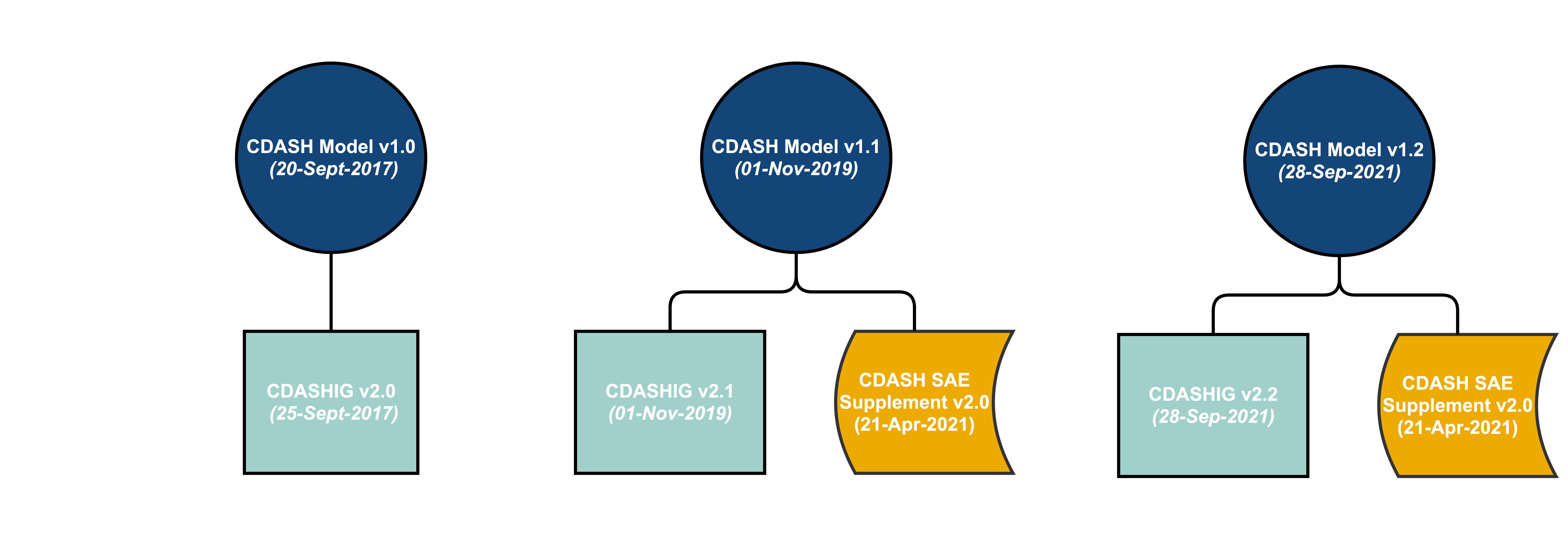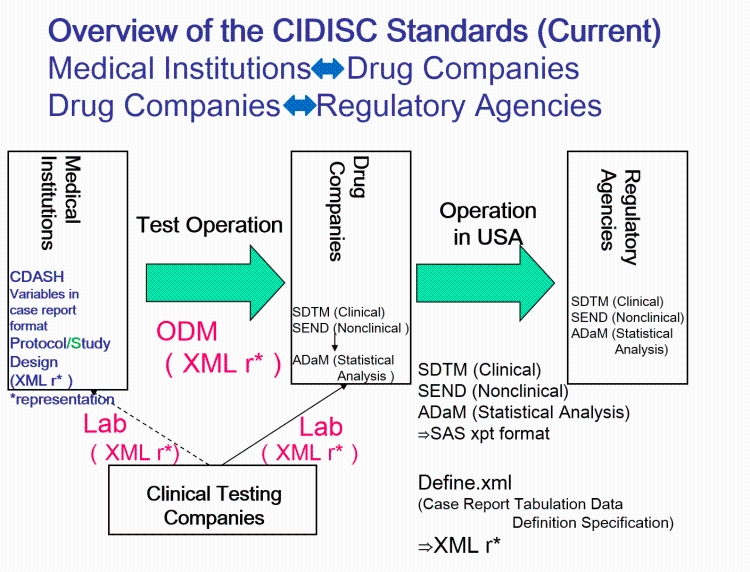
Joining the Clinical Data Interchange Standards Consortium (CDISC) and Activity Status | Japan Agency for Medical Research and Development

A pragmatic method for transforming clinical research data from the research electronic data capture “REDCap” to Clinical Data Interchange Standards Consortium (CDISC) Study Data Tabulation Model (SDTM): Development and evaluation of REDCap2SDTM -

OpenClinica becomes Gold Member with the Clinical Data Interchange Standards Consortium (CDISC) » OpenClinica

Standardizing data exchange for clinical research protocols and case report forms: An assessment of the suitability of the Clinical Data Interchange Standards Consortium (CDISC) Operational Data Model (ODM) - ScienceDirect
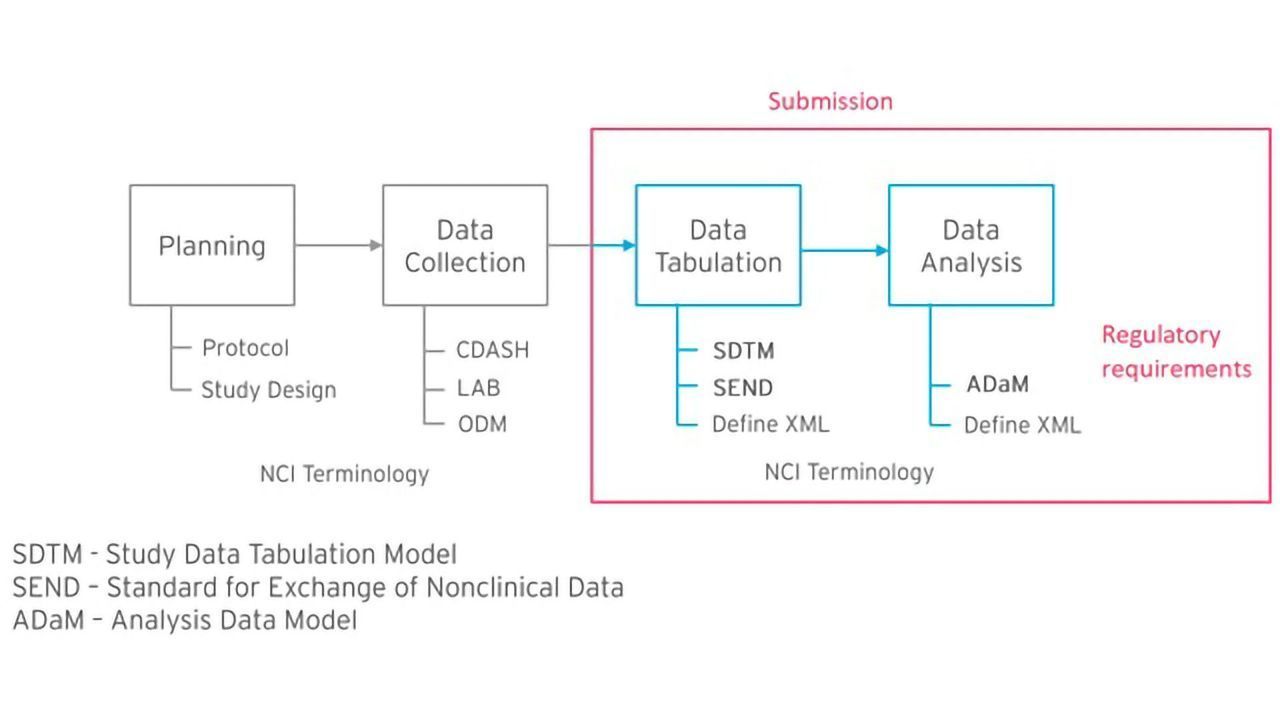




.png)