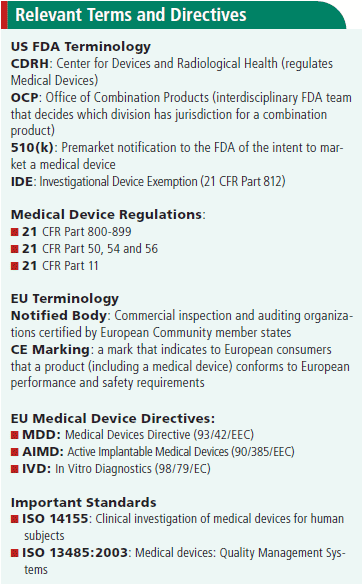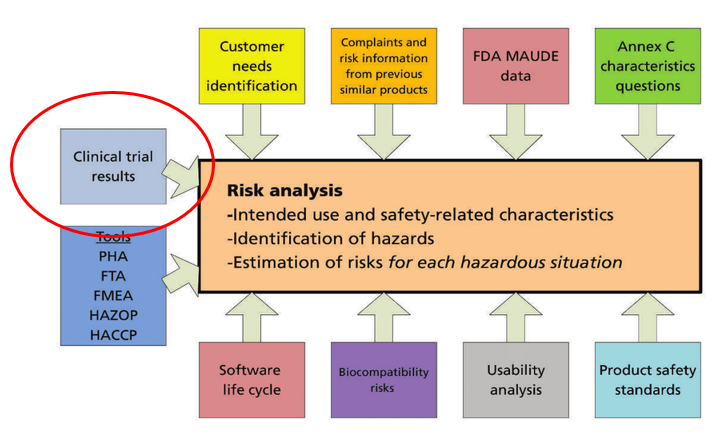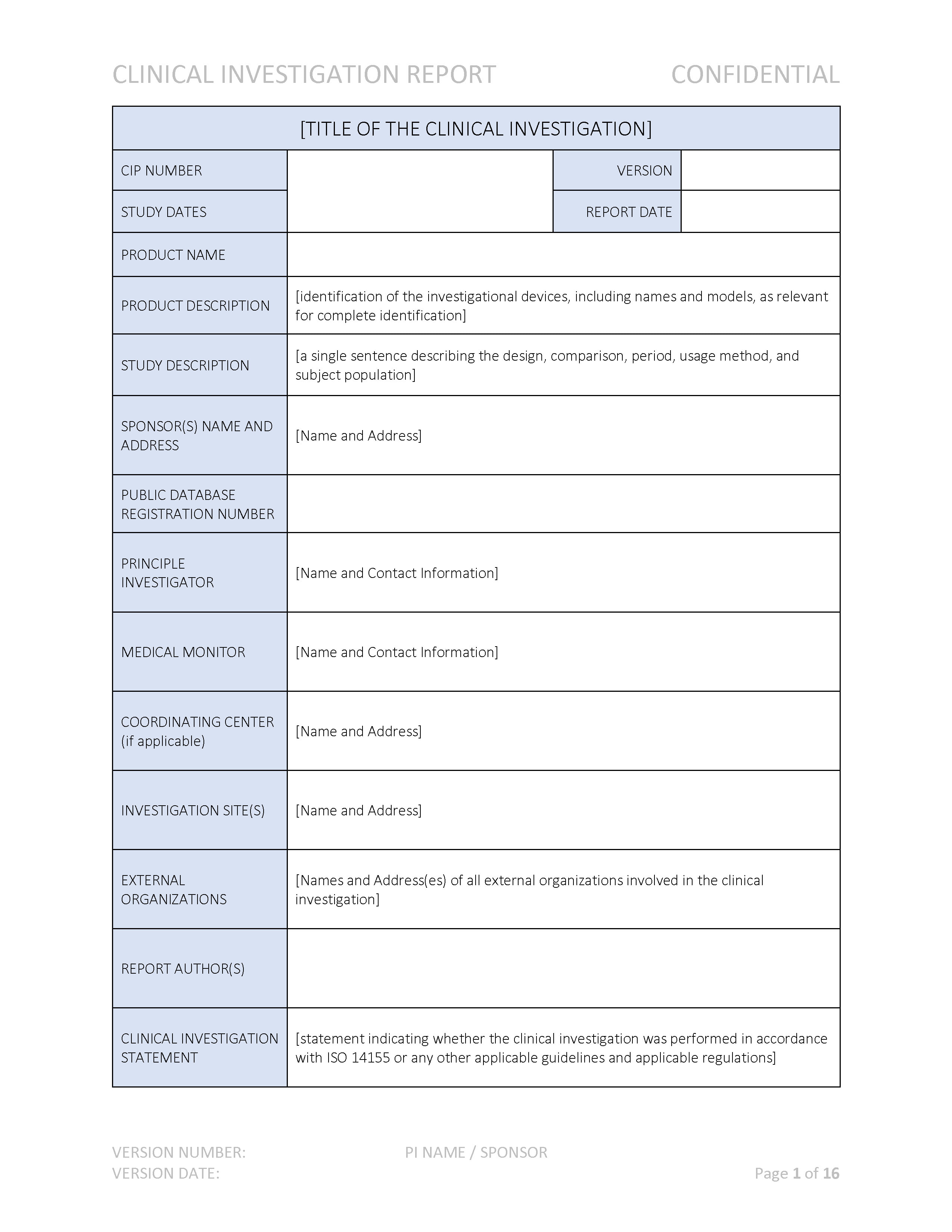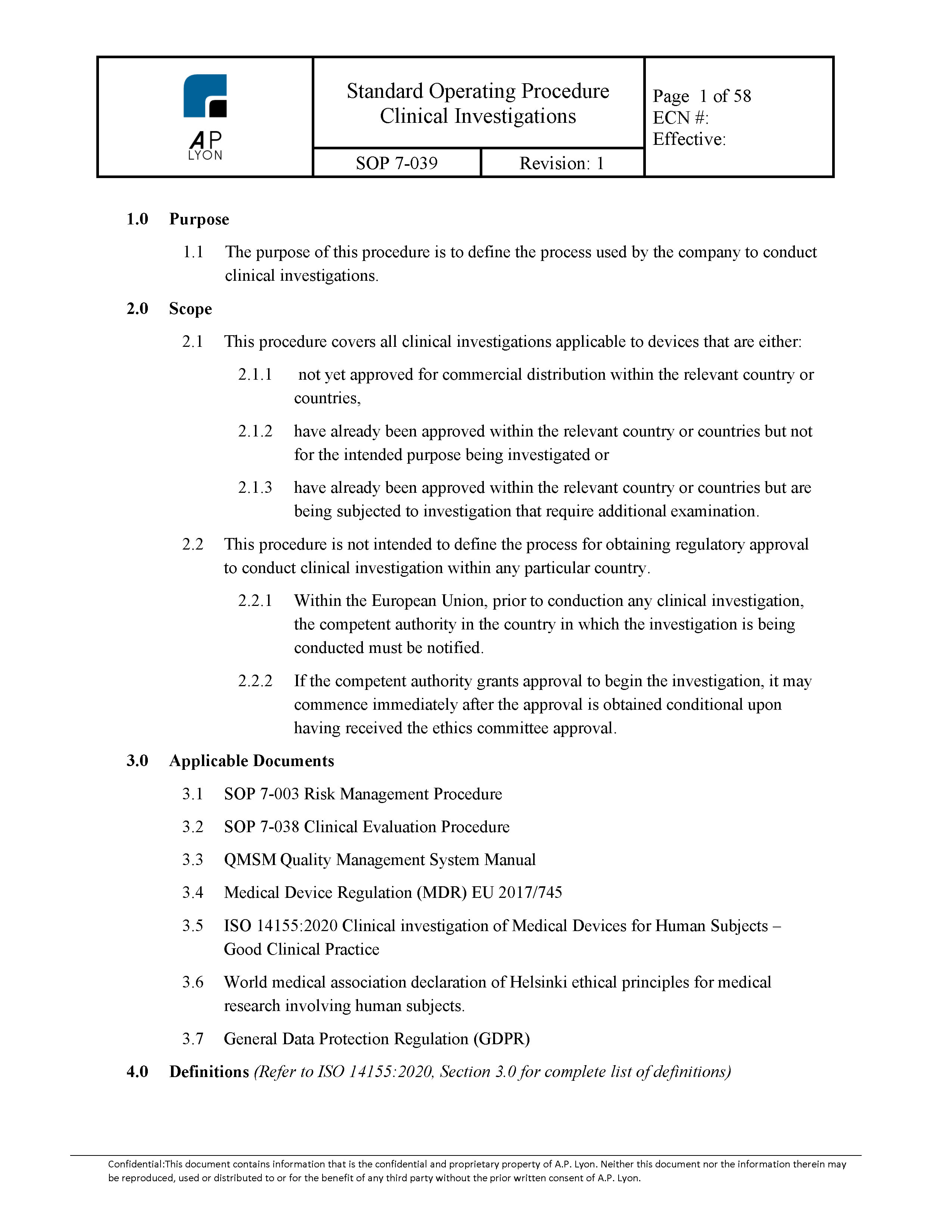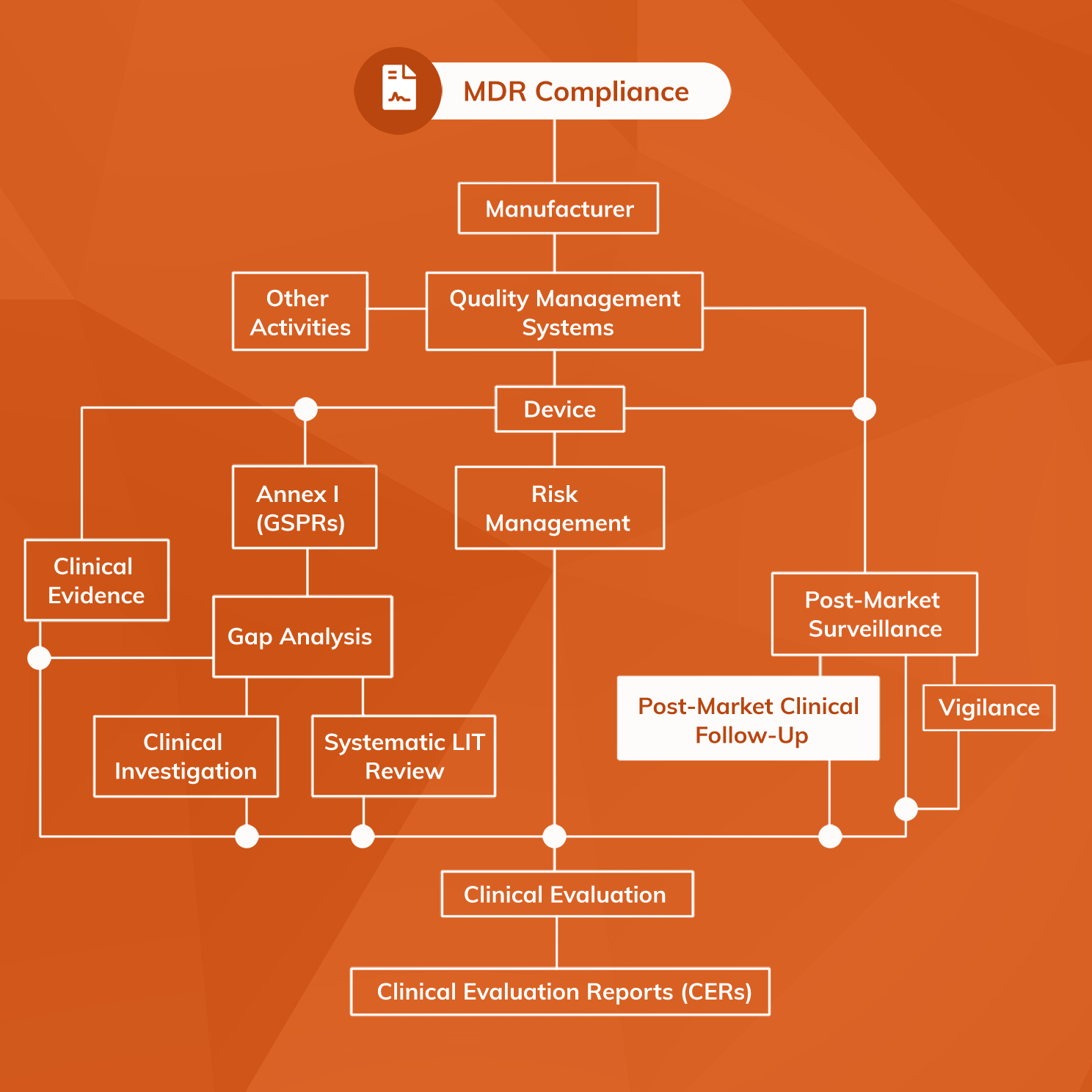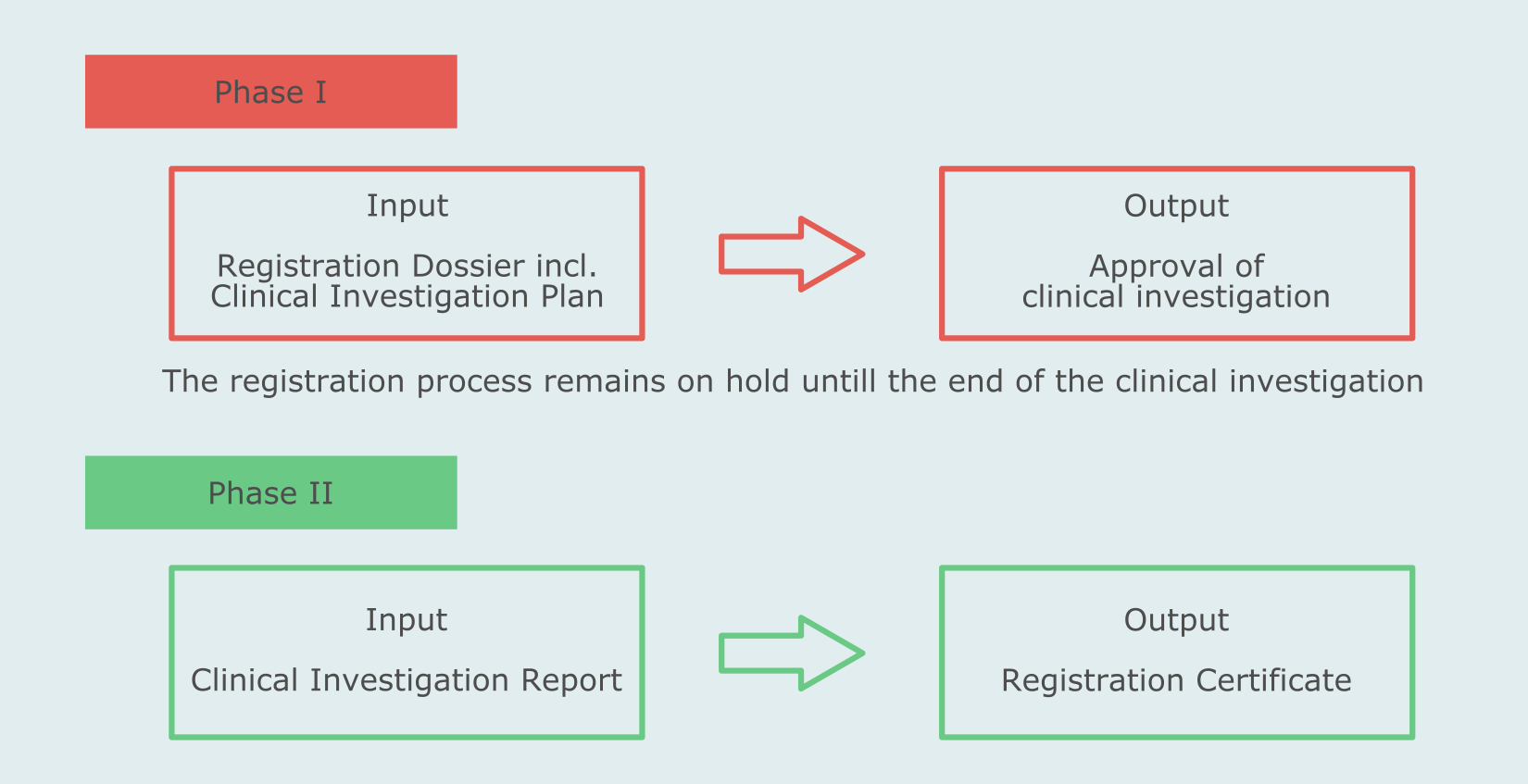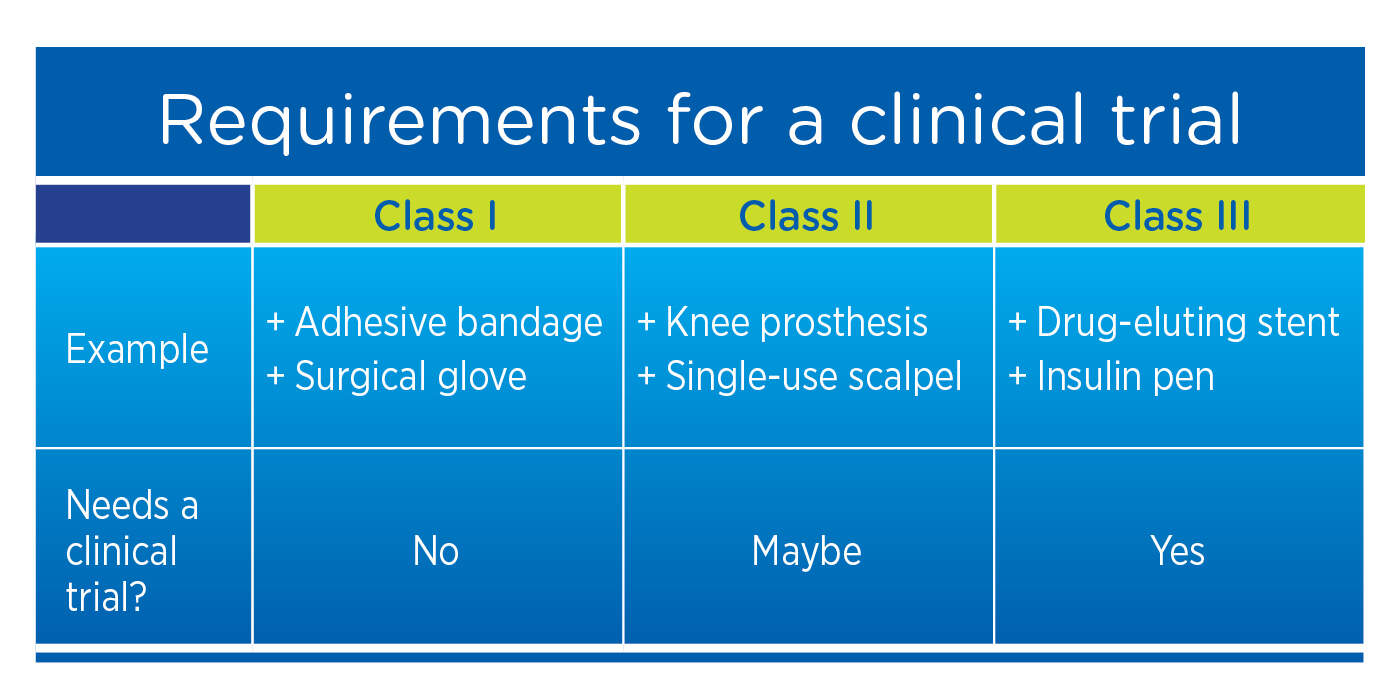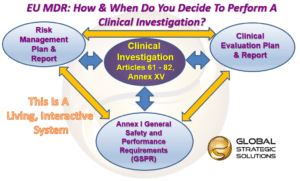
What Is The Difference Between Clinical Evaluation and Clinical Investigation? | Global Strategic Solutions
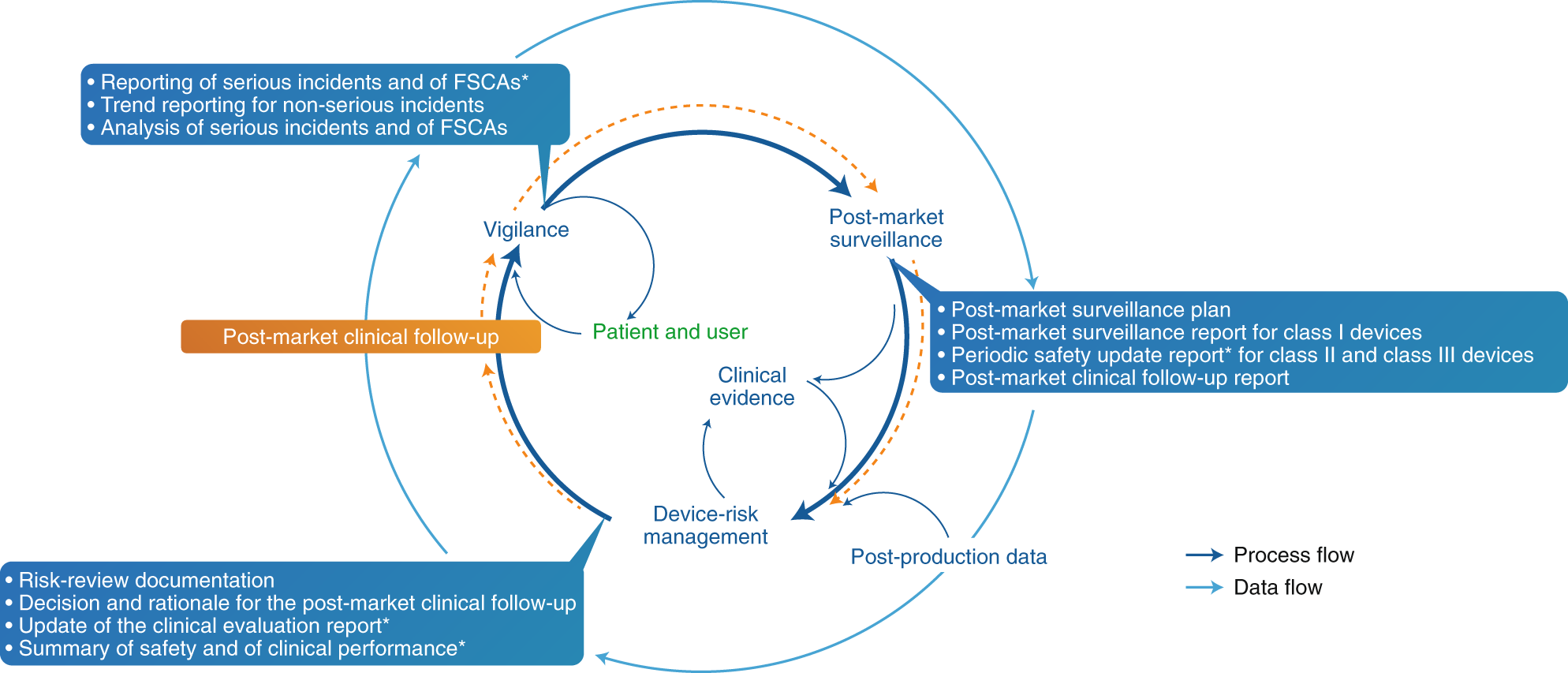
How the new European regulation on medical devices will affect innovation | Nature Biomedical Engineering

Medical Device Quality, Regulatory and Product Development Blog | Greenlight Guru | QMS Software (4)

Importance of systematic literature search for clinical evaluation(ce) the strict adherence of medde by PepGra CRO - Issuu