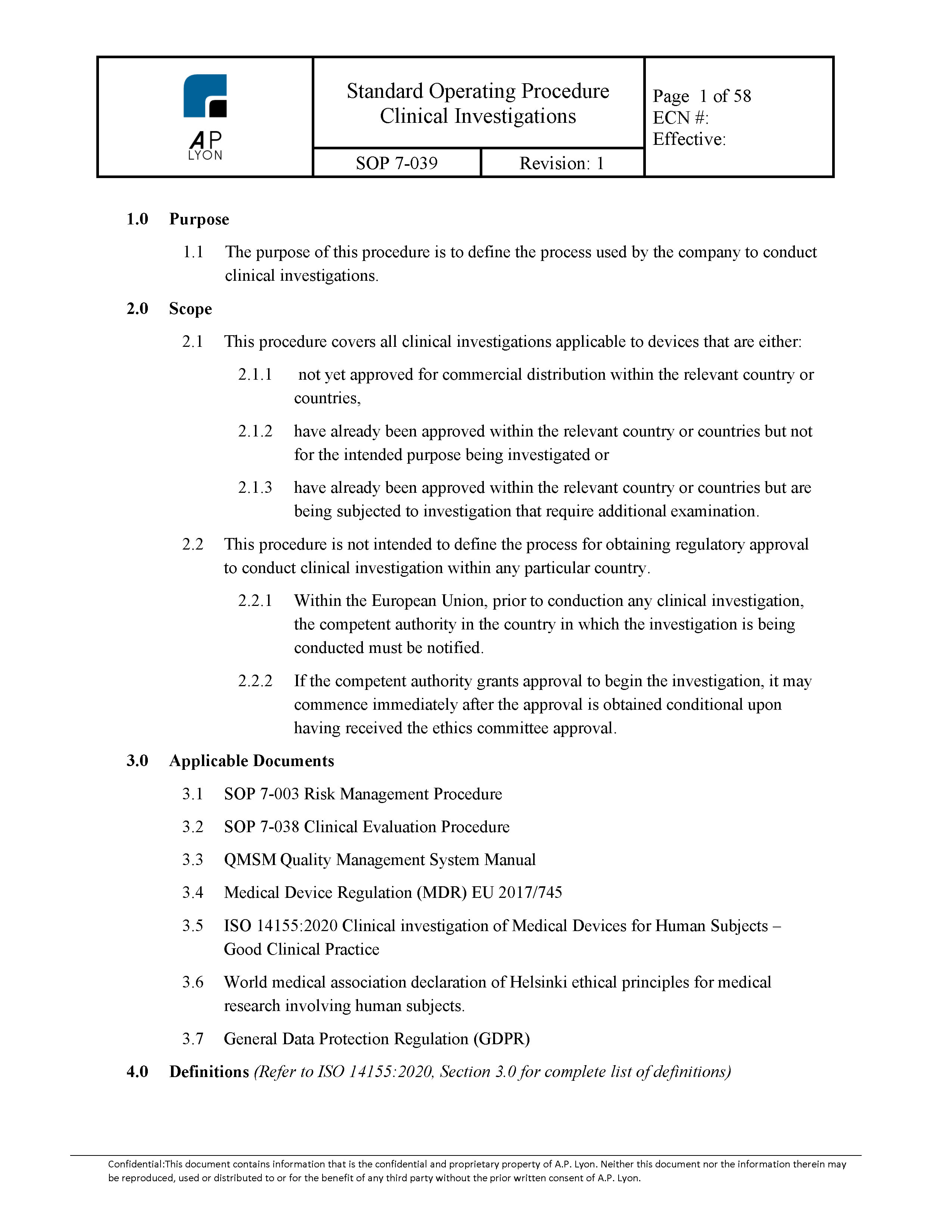
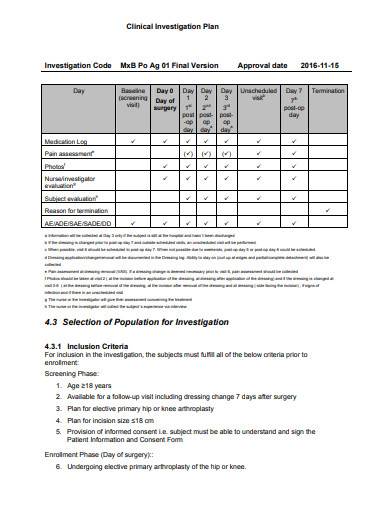
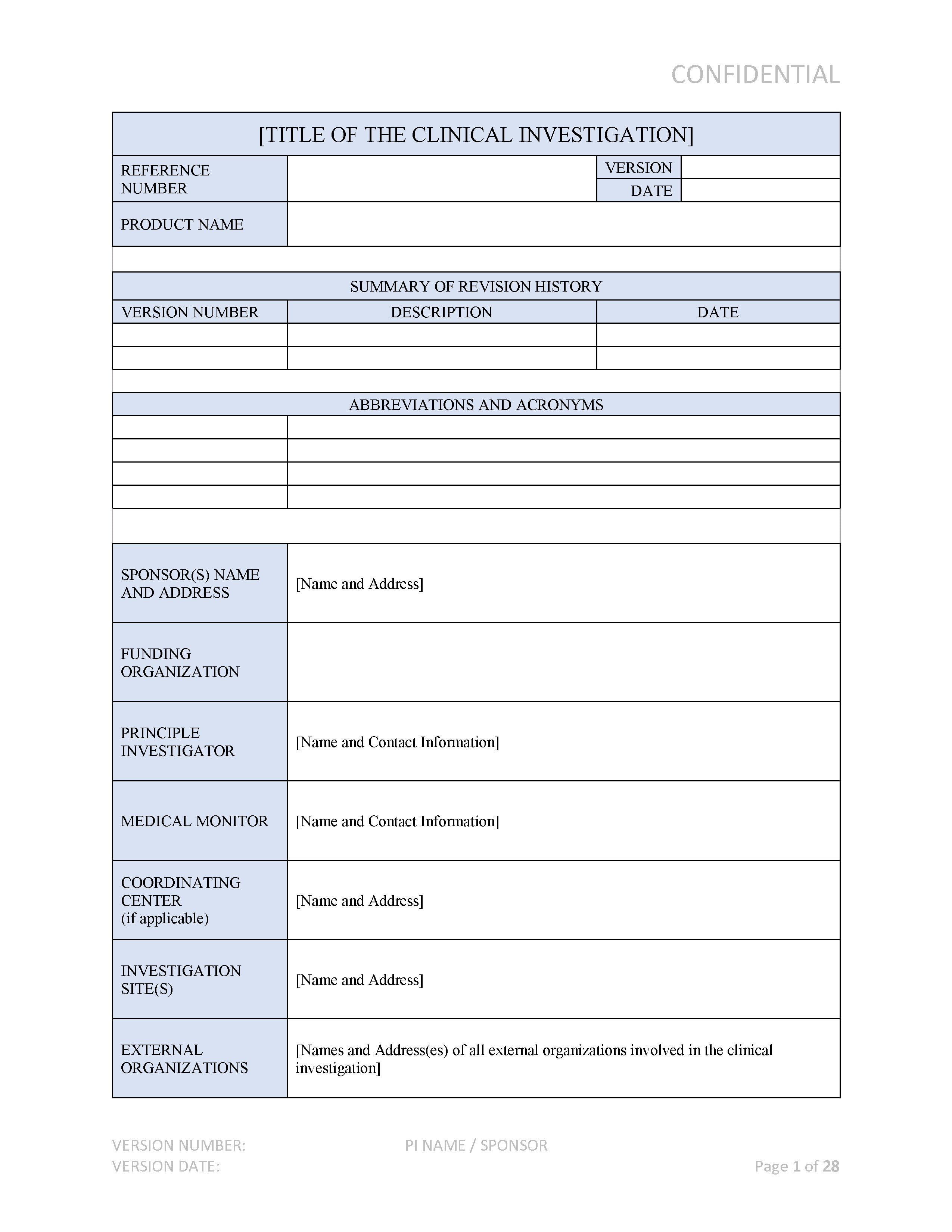
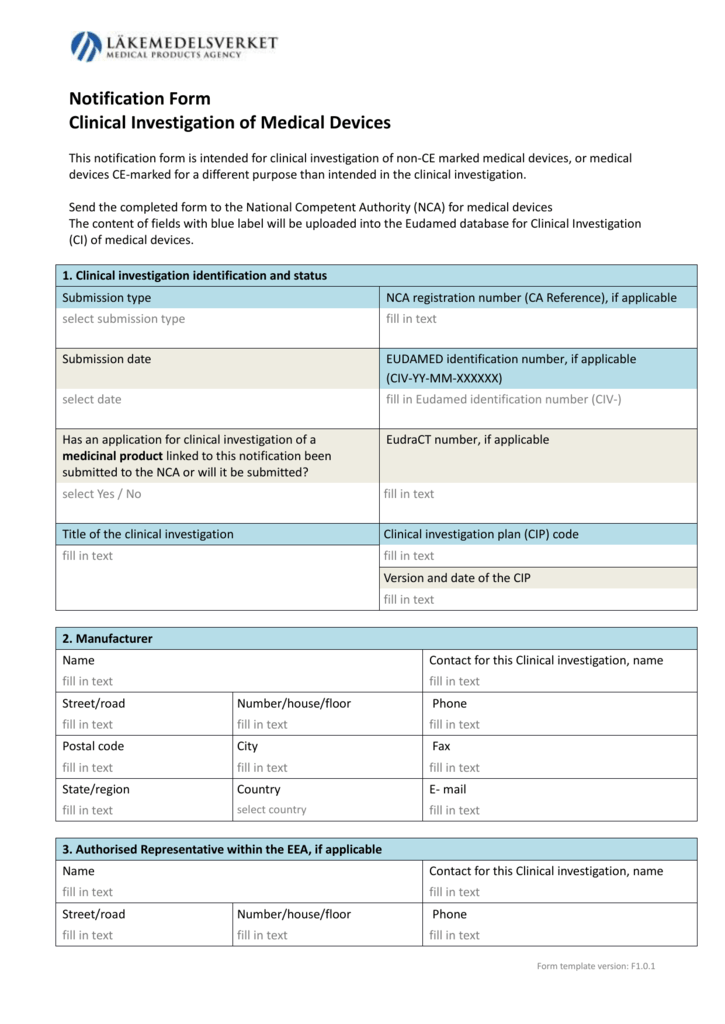
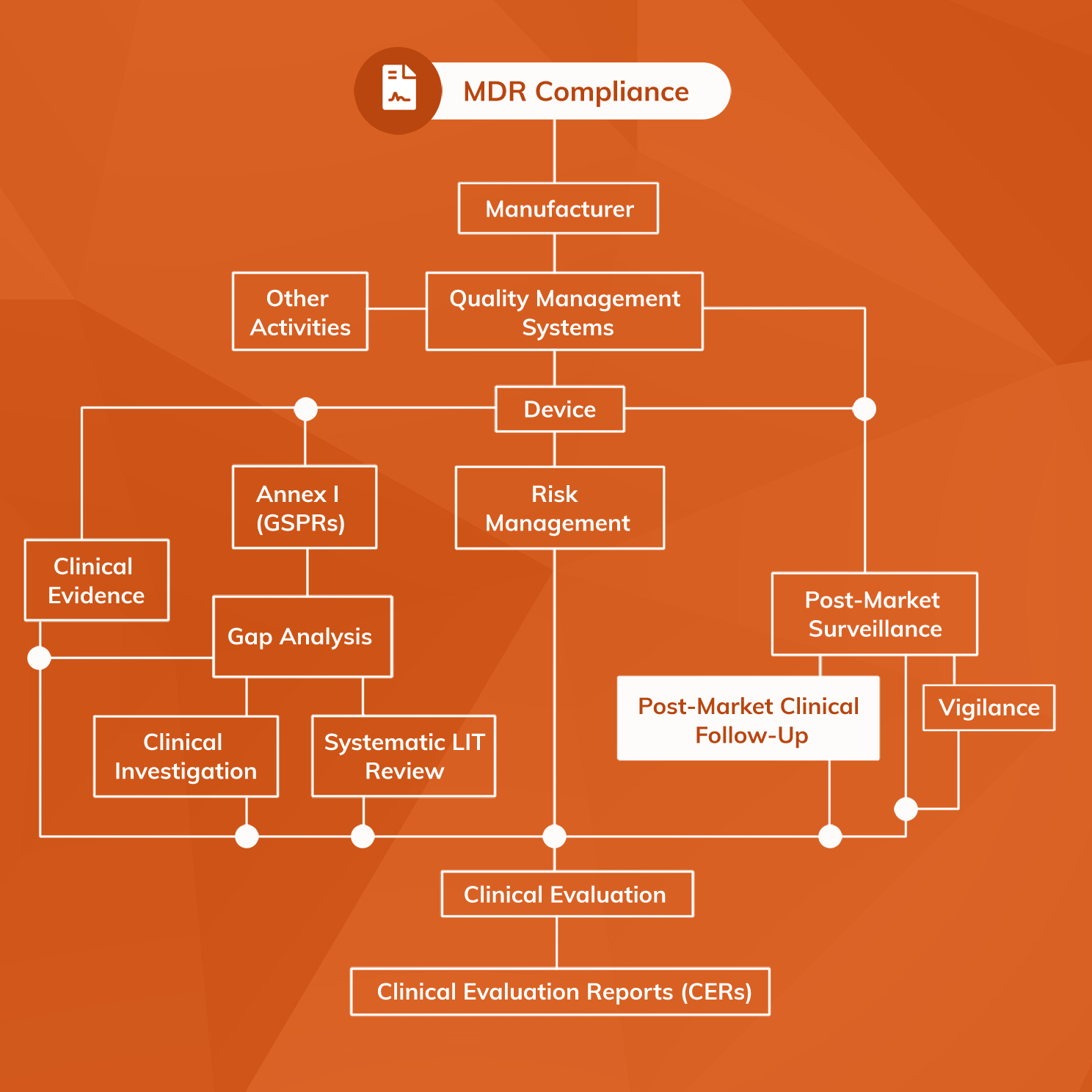
What you Need and When – The Key Documents in the Drug Lifecycle - Trilogy Writing & Consulting GmbH
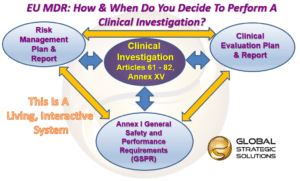
What Is The Difference Between Clinical Evaluation and Clinical Investigation? | Global Strategic Solutions

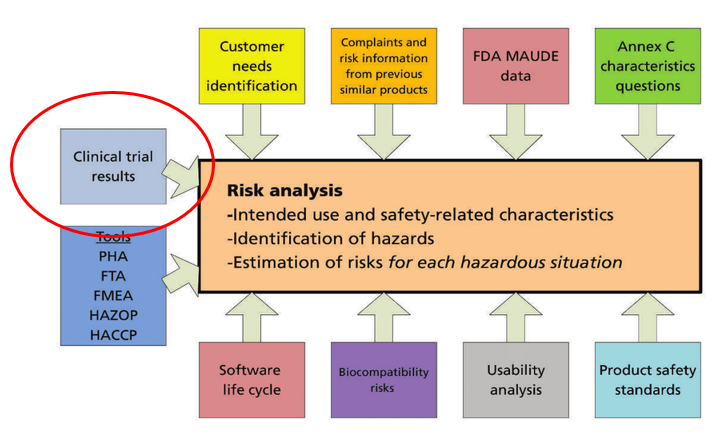
Current EU and Global Activities in the „Clinicals“ Dr. Wolfgang Ecker Federal Ministry of Health, Austria Chairman of EU WG CIE 3rd National Conference. - ppt download