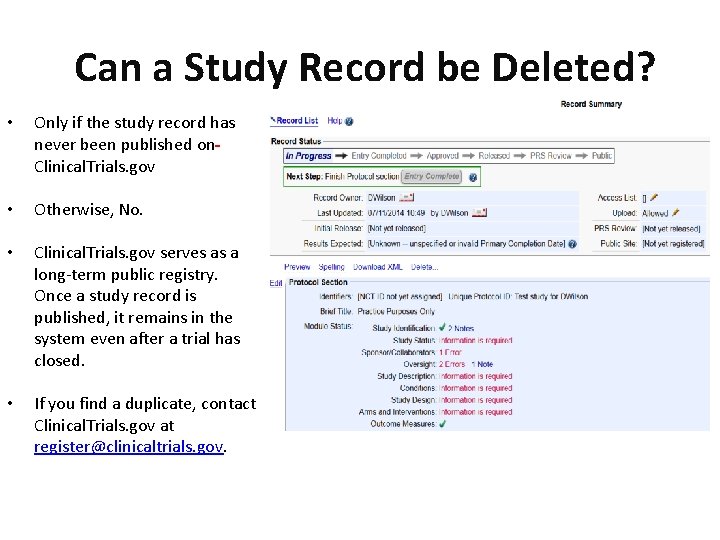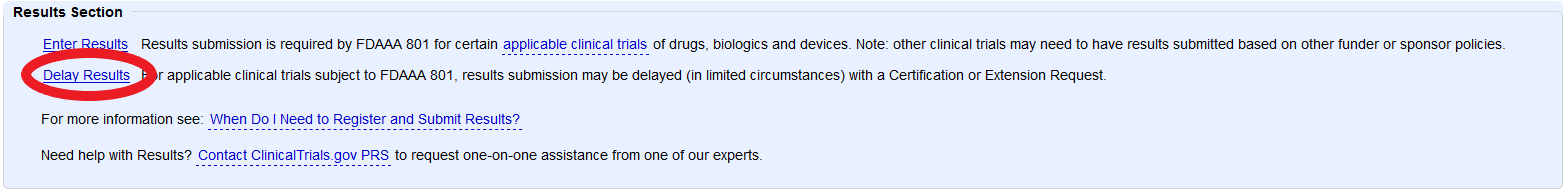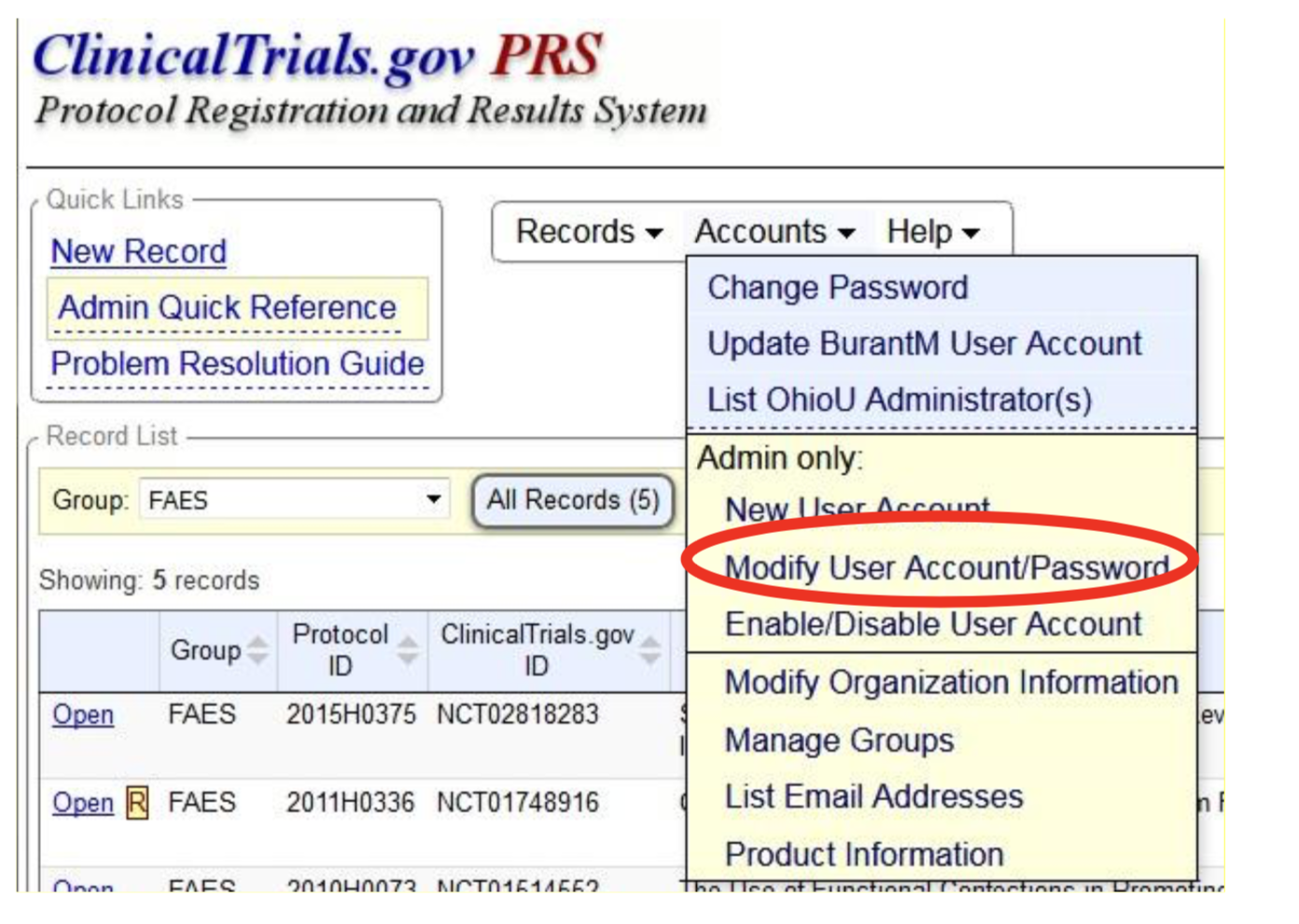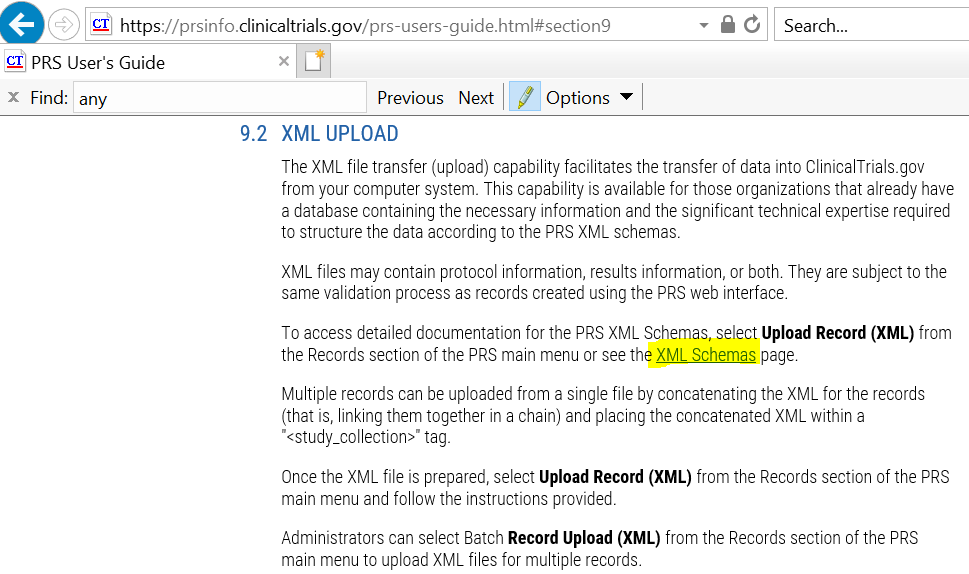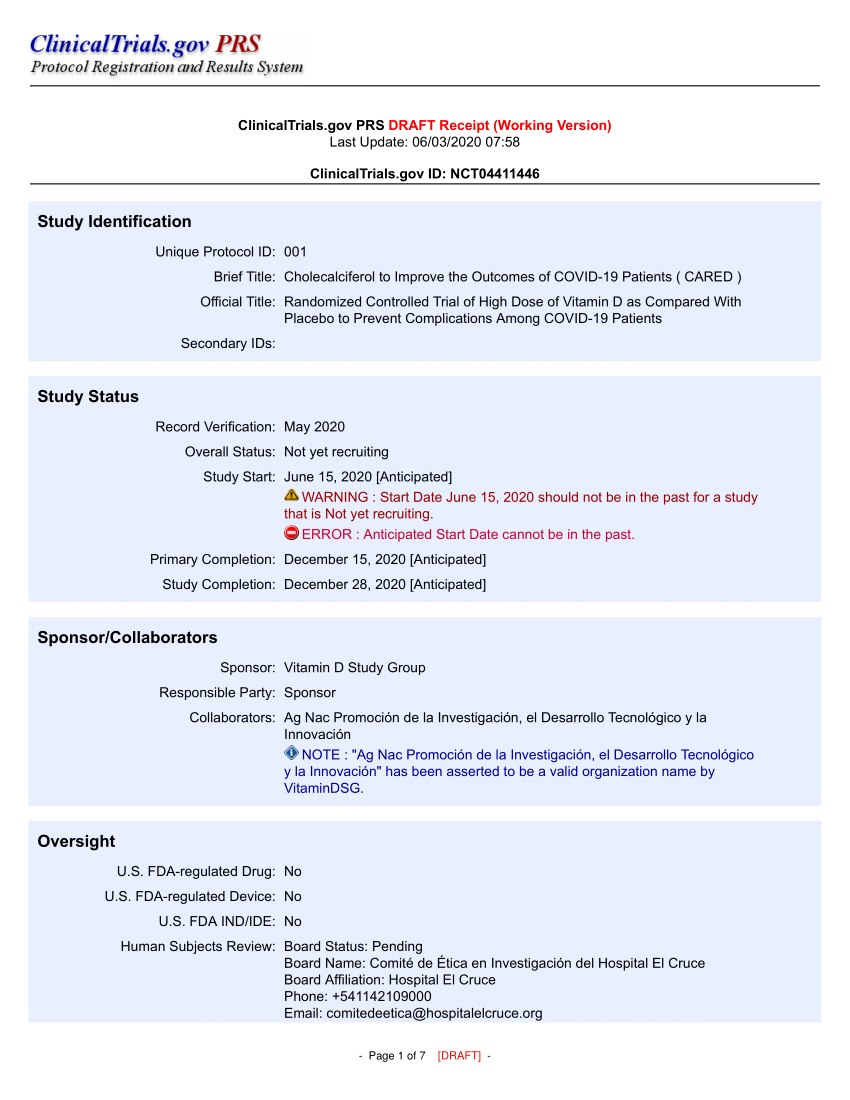
PDF) ClinicalTrials.gov Brief Title: Cholecalciferol to Improve the Outcomes of COVID-19 Patients ( CARED ) Official Title: Randomized Controlled Trial of High Dose of Vitamin D as Compared With Placebo to Prevent

ClinicalTrials.gov: How to Register Your Trial - Clinical and Translational Science Institute - University at Buffalo

PDF) 3381 Reducing Problem Records in the Johns Hopkins University ClinicalTrials.gov Protocol Registration and Results System (PRS)

Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study - The Lancet