Current clinical trial approval process in India. Abbreviations: BM,... | Download Scientific Diagram

Data Safety and Monitoring Boards Should Be Required for Both Early- and Late-Phase Clinical Trials - ScienceDirect
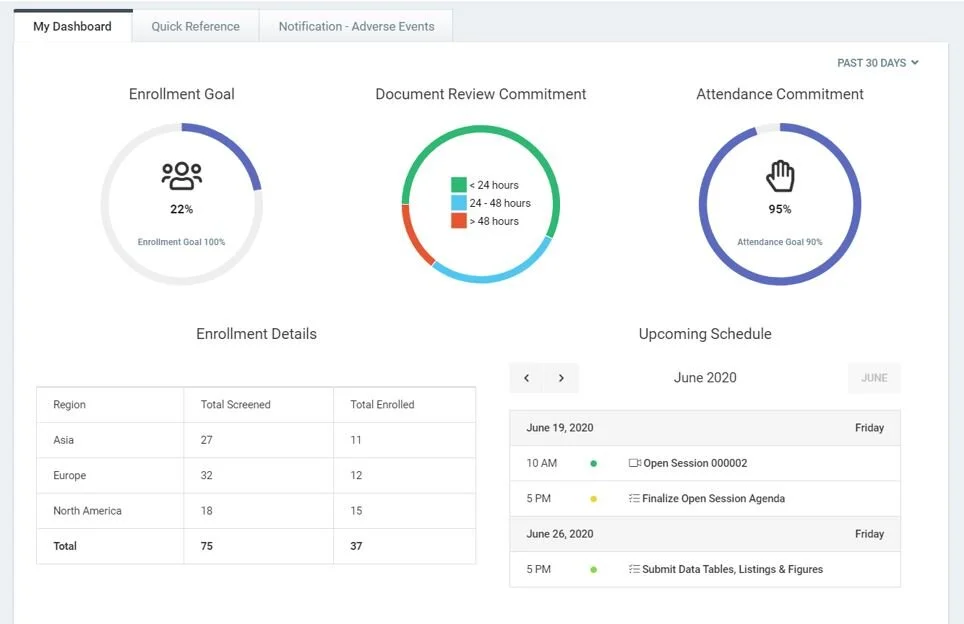
Clinical Trial Oversight | DSMB Software | Data Monitoring Committee in Clinical Trials | Cloud Concinnity®
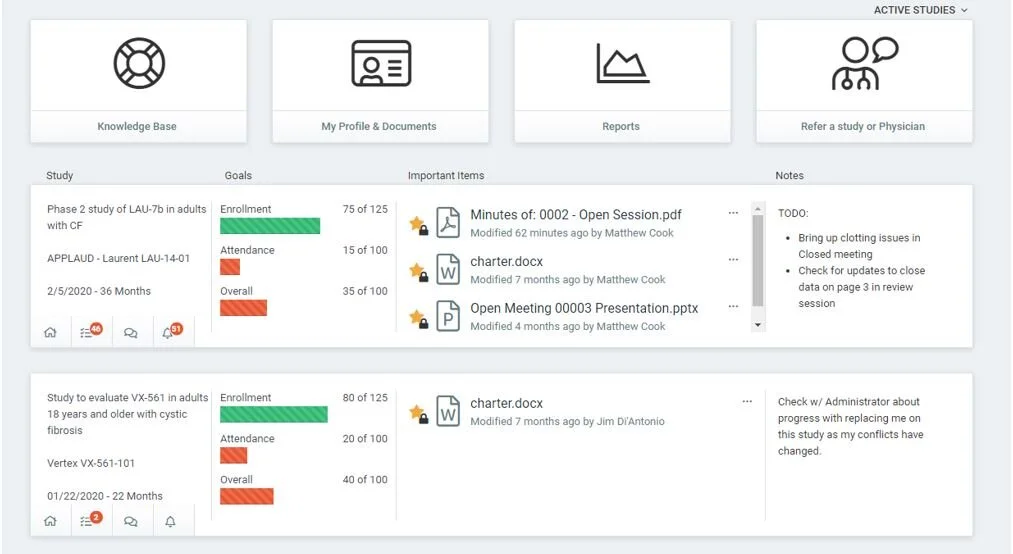
Clinical Trial Oversight | DSMB Software | Data Monitoring Committee in Clinical Trials | Cloud Concinnity®
Clinical Trial Oversight | DSMB Software | Data Monitoring Committee in Clinical Trials | Cloud Concinnity®

The charming Ppt – Data And Safety Monitoring In Clinical Trials Within Intended For Monitoring Report Template Clinical… | Report template, Clinical trials, Trials

The breathtaking Dsmb Report Form Template Pertaining To Trial Report Template digital photography below… | Report template, Professional templates, Clinical trials
Traditional centralized system of data management and communication in... | Download Scientific Diagram

Guidelines for Developing a Data and Safety Monitoring Plan | National Institute on Drug Abuse (NIDA)

Reporting of data monitoring boards in publications of randomized clinical trials is often deficient: ACTTION systematic review - Journal of Clinical Epidemiology