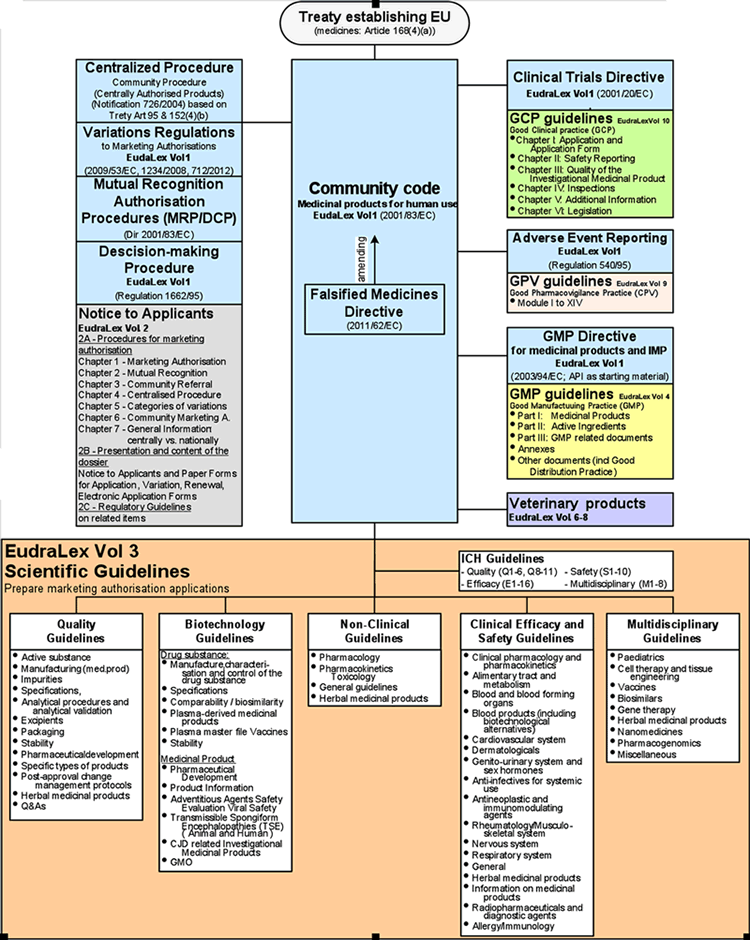
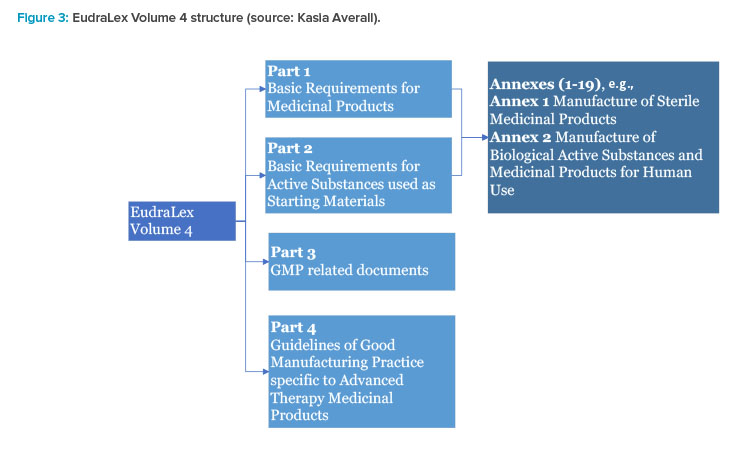
EudraLex - Volume 4 - Good Manufacturing Practice (GMP) guidelines 2017 - Free PDF Download | M A N O X B L O G

EudraLex - Volume 4 - Good Manufacturing Practice (GMP) guidelines - Free PDF download | M A N O X B L O G

EudraLex - Volume 4 - Good Manufacturing Practice (GMP) guidelines 2017 - Free PDF Download | M A N O X B L O G
EMA EudraLex - Volume 4 - GMP Guidelines - TELUGU GMP - Provides GMP Pharmaceutical Guidelines in Telugu.

Eudralex Volume 3B: Guidelines: Medicinal Products for Human Use - Safety, Environment and Information by European Communities: Near Fine Soft cover (1998) | The Book Exchange