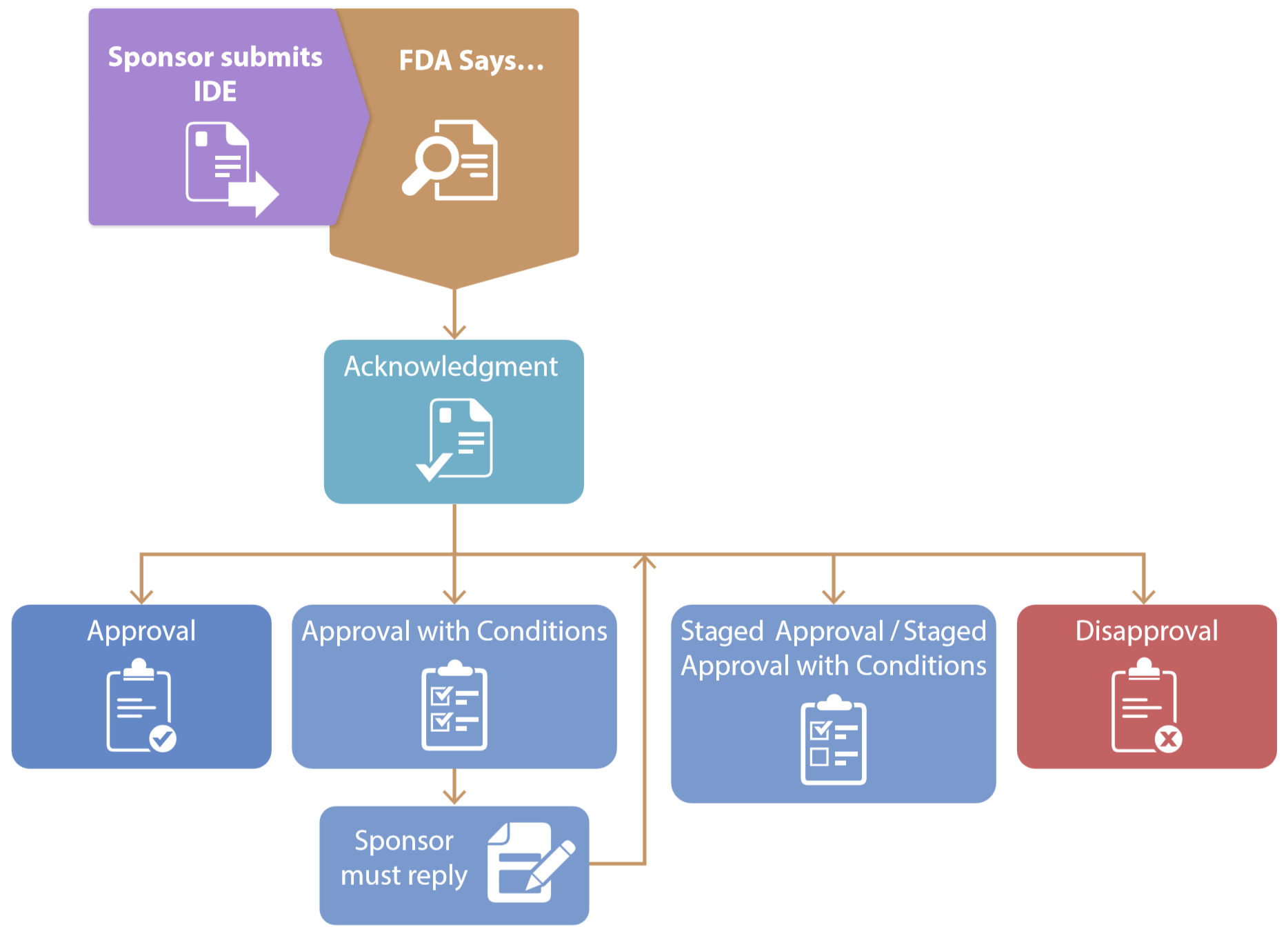
FDA Decisions for Investigational Device Exemption Clinical Investigations - Guidance for Sponsors, Clinical Investigators, Inst
Live Case Presentations During Investigational Device Exemption (IDE) Clinical Trials - Guidance for Institutional Review Boards
FDA Decisions for Investigational Device Exemption Clinical Investigations - Guidance for Sponsors, Clinical Investigators, Inst
IDE DECISION WORKSHEET For Investigator-Initiated Clinical Investigations Does Your Study Require an IDE Submittal to the FDA? N
FDA Categorization of Investigational Device Exemption (IDE) Devices to Assist the Centers for Medicare and Medicaid Services (C

FDA Regulation of Neurological and Physical Medicine Devices: Access to Safe and Effective Neurotechnologies for All Americans - ScienceDirect
FDA Categorization of Investigational Device Exemption (IDE) Devices to Assist the Centers for Medicare and Medicaid Services (C
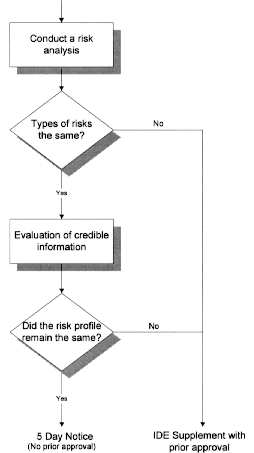
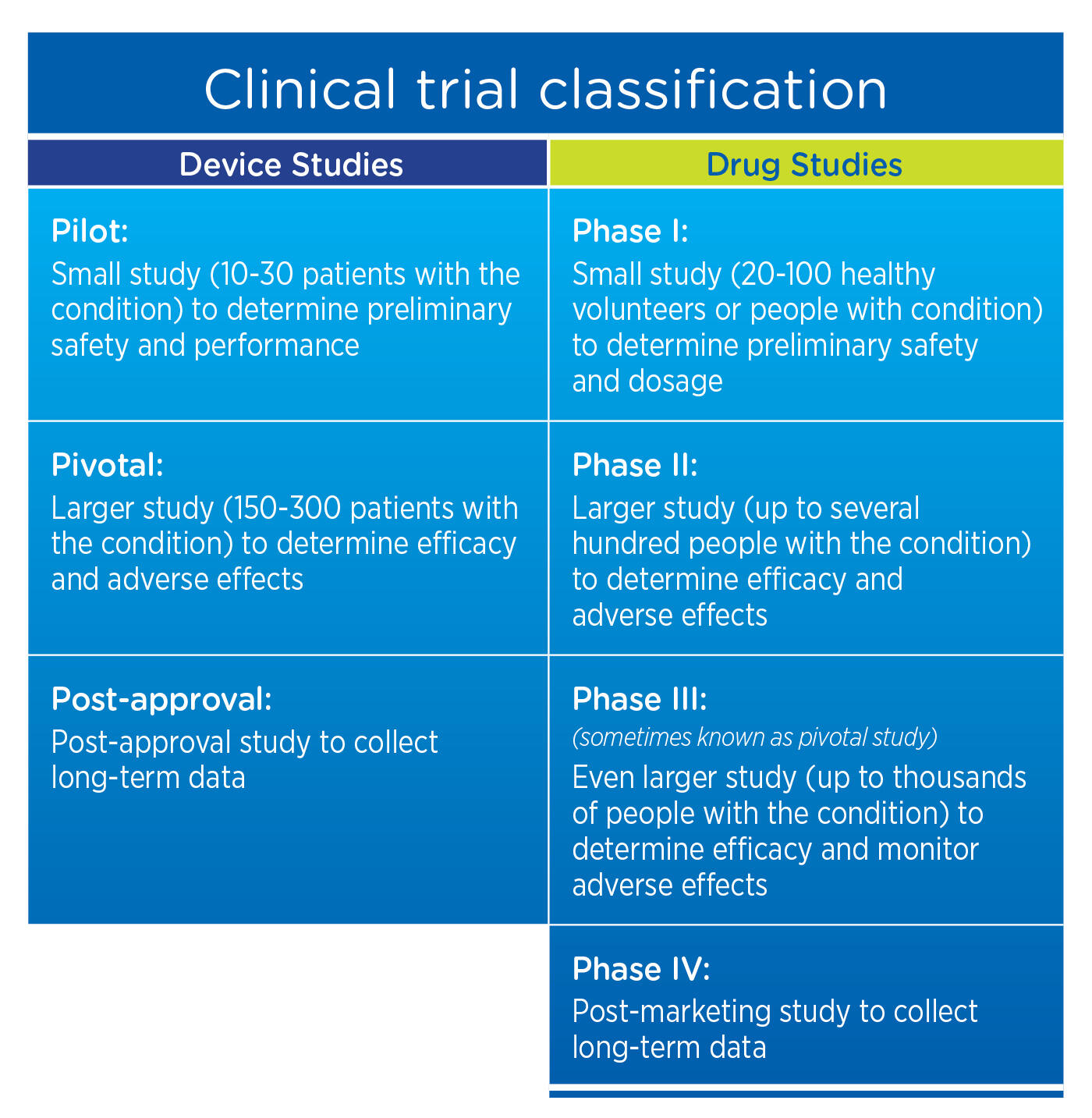
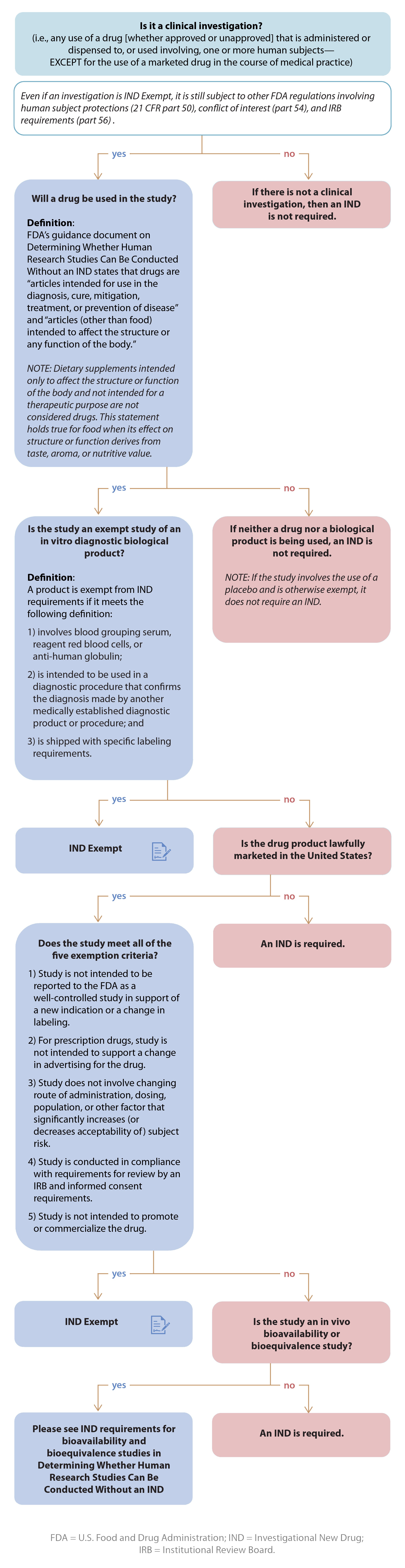
Page 1 of 5 Title: Requirements for Investigational Device Exemption (IDE) for Human Subject Research Department: Human Research
Guidance and Procedures: Use of Devices in Clinical Research and Brief Overview Investigational Device Exemption Applications (