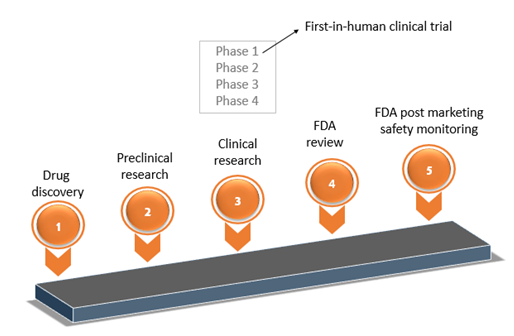
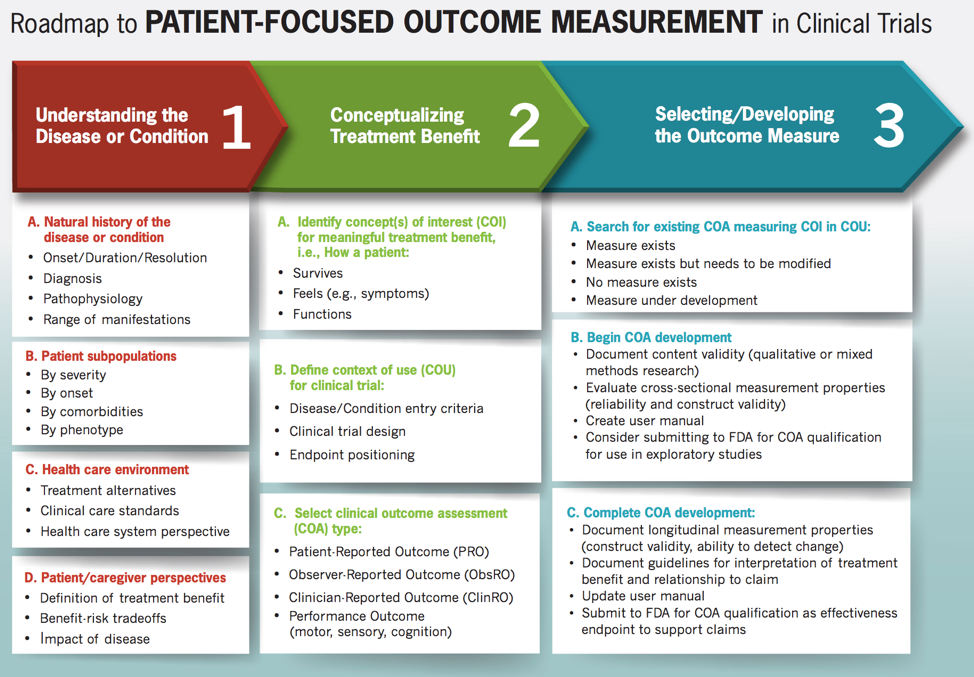
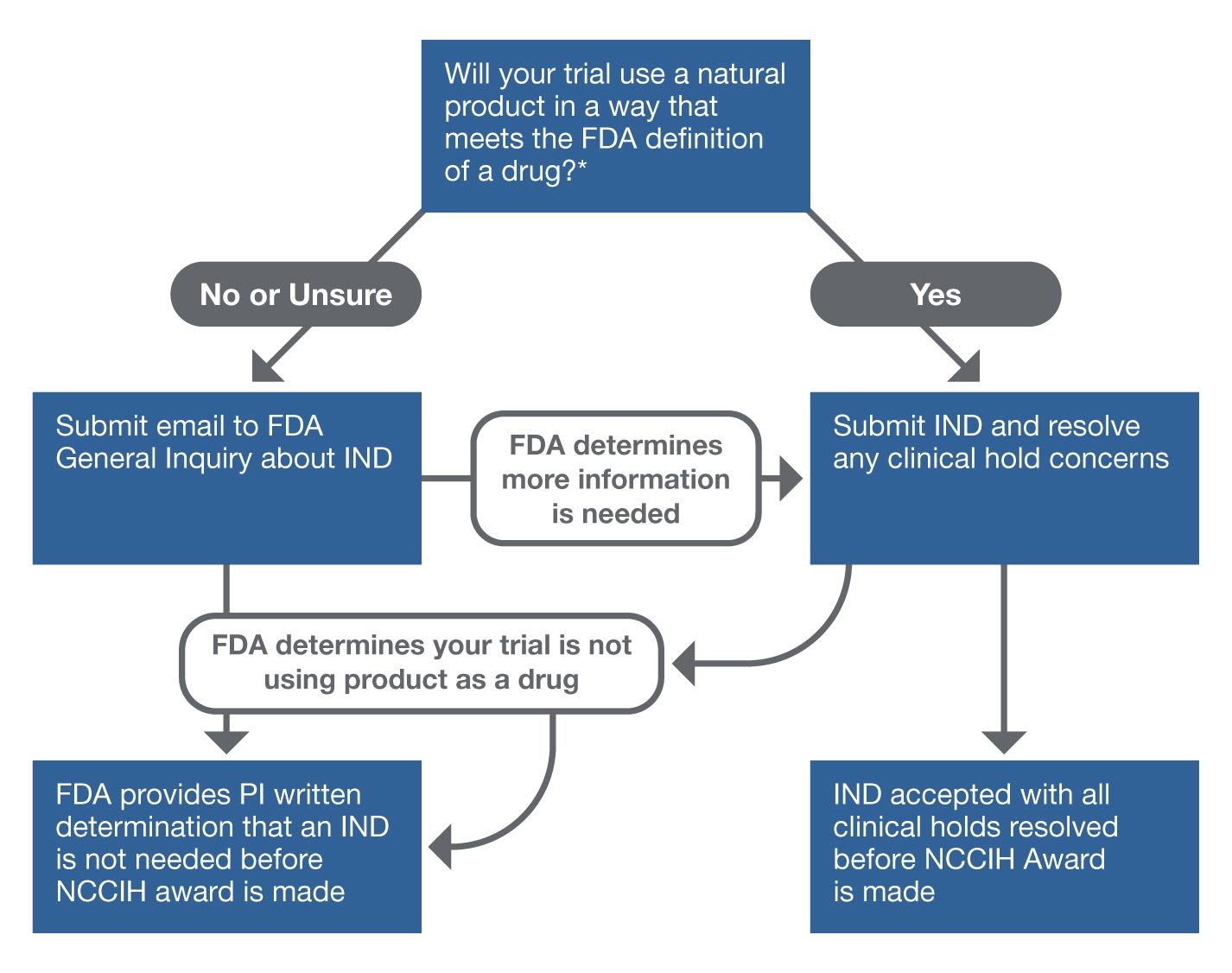
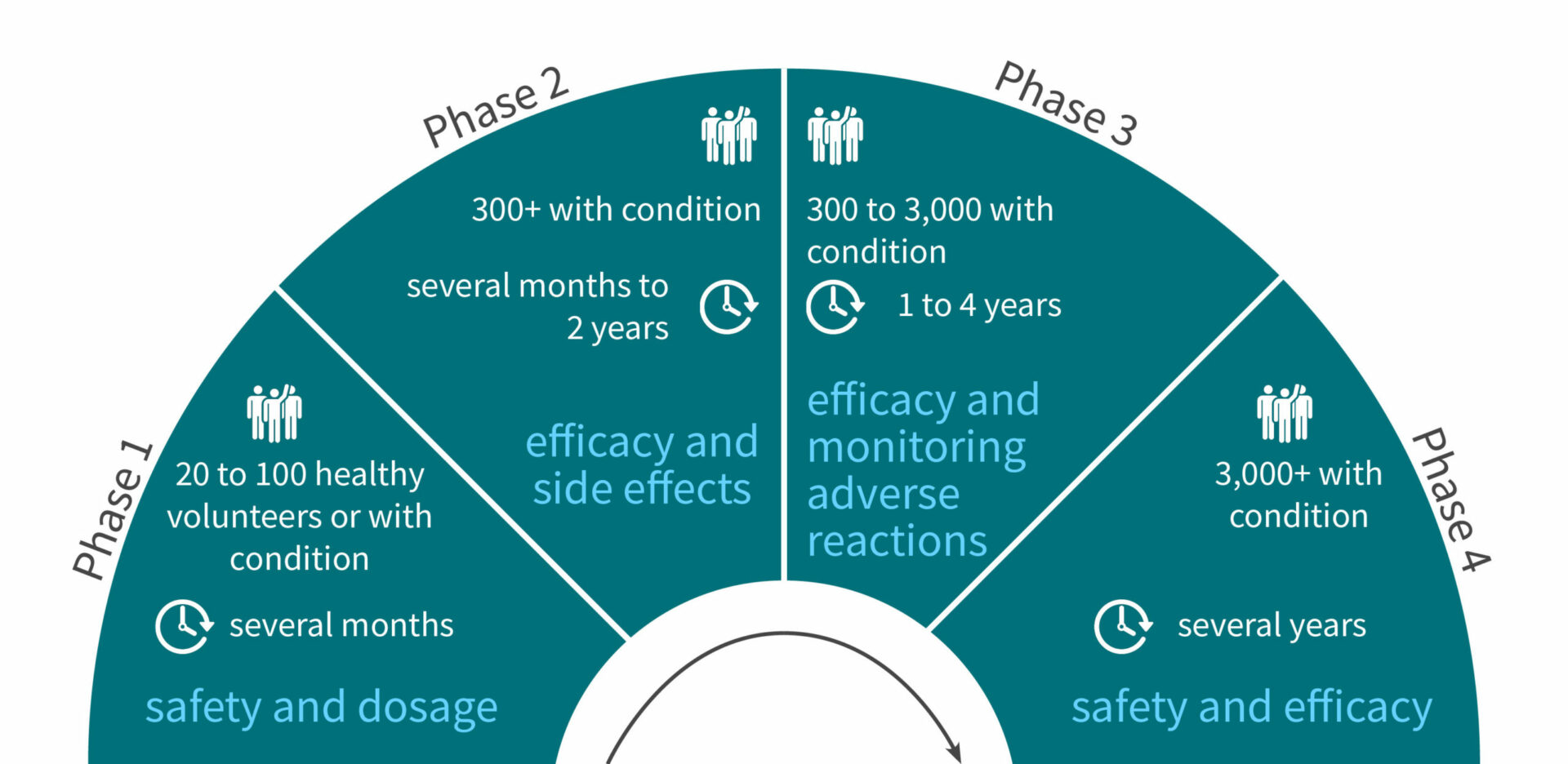
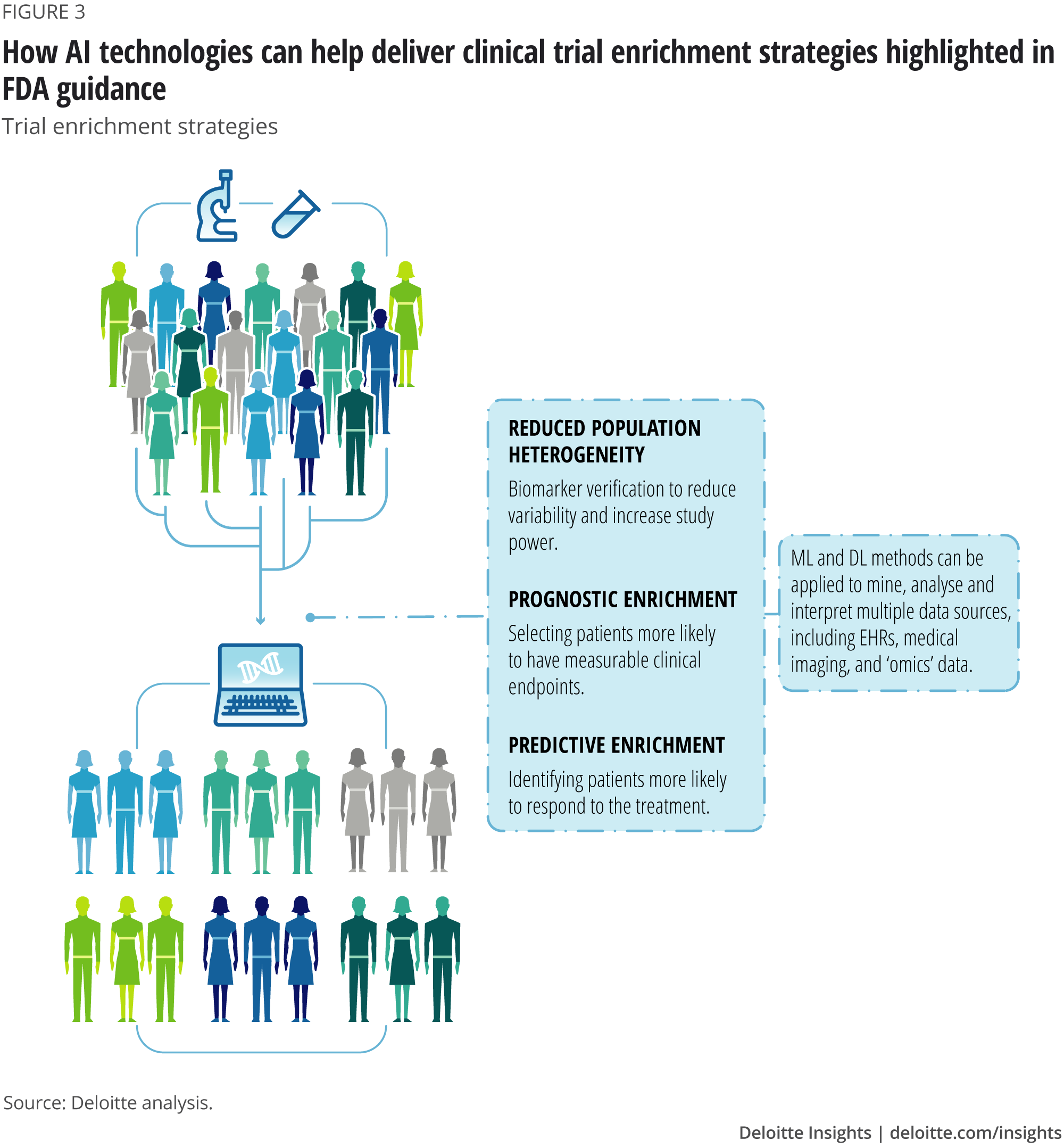
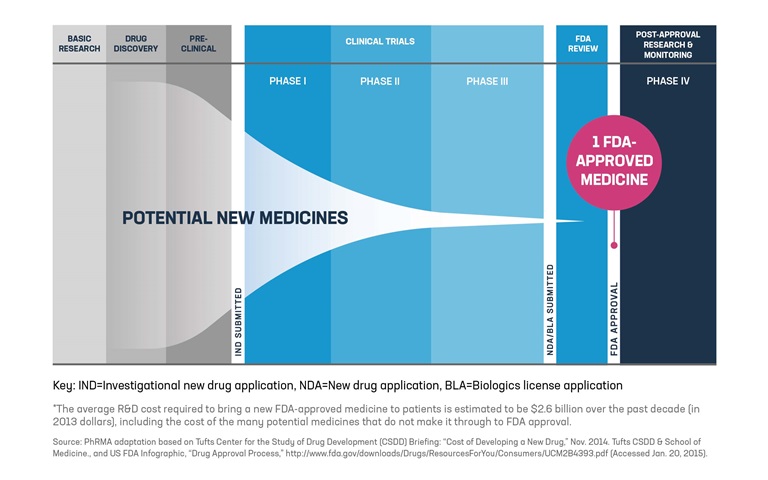
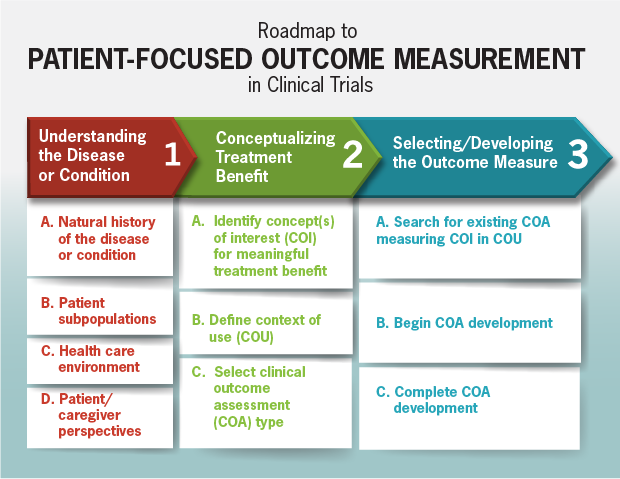
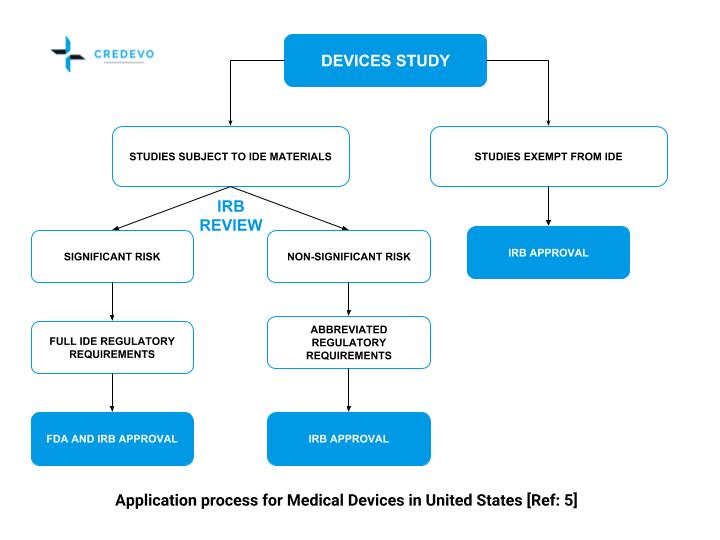
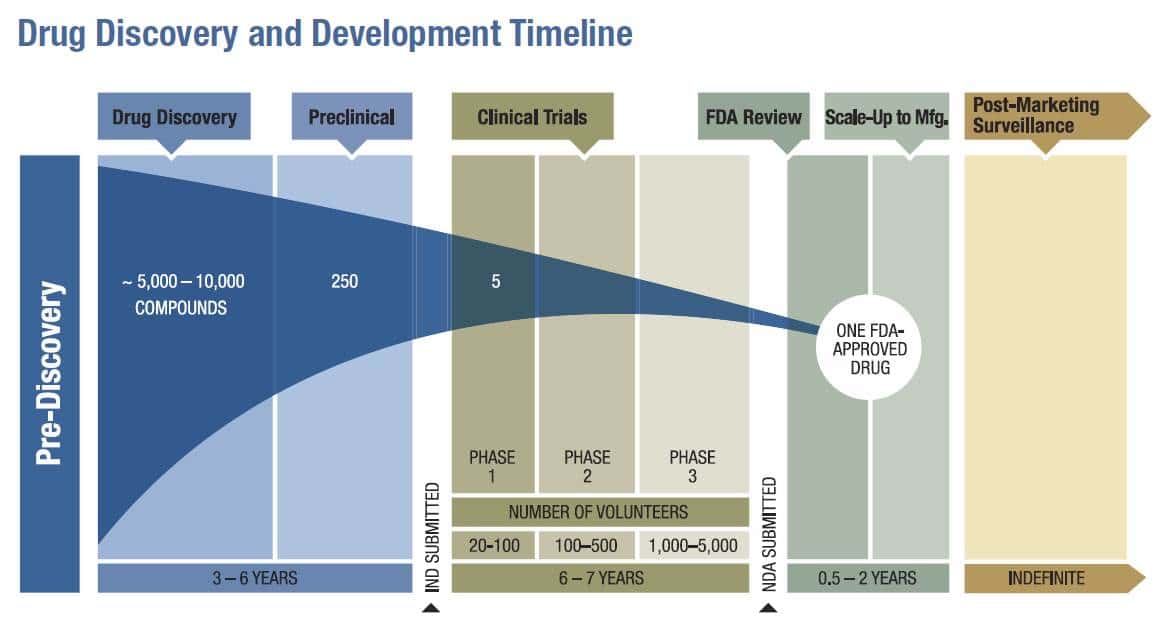
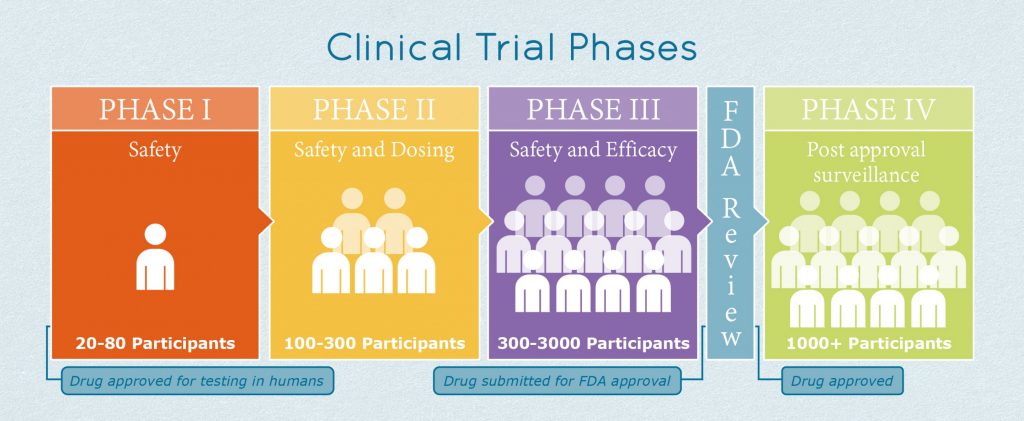
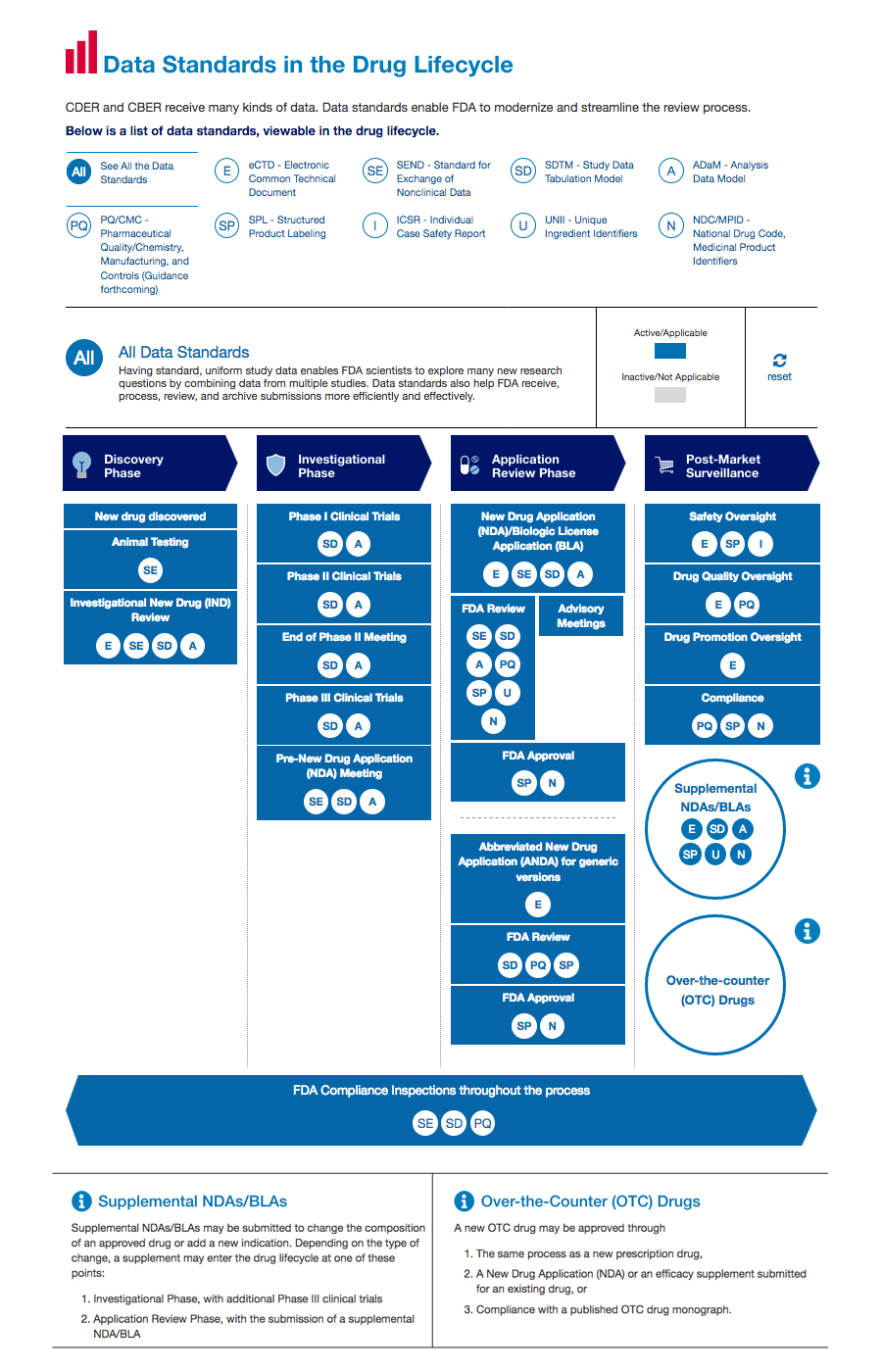
FDA roadmap to patient-focused outcome measurement in clinical trials. 3 | Download Scientific Diagram

FDA Clears Up Remote Data Use In Clinical Trials With New Guidance | The Healthcare Technology Report.

Investigational New Drugs: FDA Has Taken Steps to Improve the Expanded Access Program but Should Further Clarify How Adverse Events Data Are Used | U.S. GAO

Clinical Trials: Study Design, Endpoints and Biomarkers, Drug Safety, and FDA and ICH Guidelines: Brody PhD, Tom: 9780128042175: Amazon.com: Books

Safety Reporting Overload in Clinical Trials: FDA and Site Perspectives on Overreporting of Adverse Events | CenterWatch