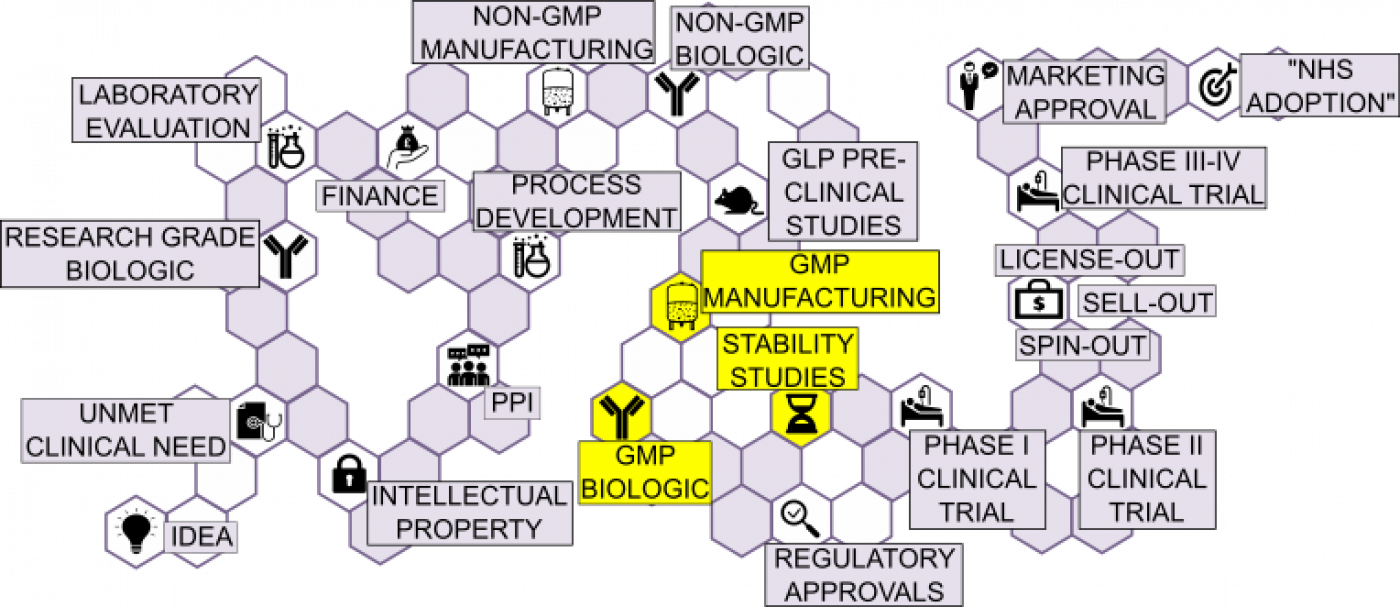
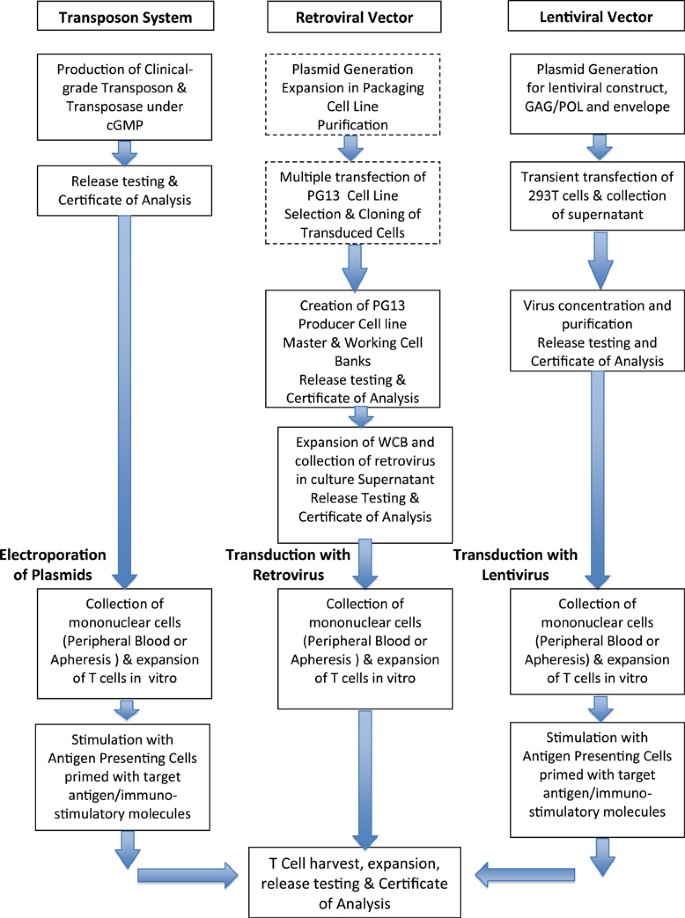
Manufacturing a Clinical Trial Product (Biologics) | UCL Therapeutic Innovation Networks - UCL – University College London

Good Manufacturing Practice in China: Equipment Strategy and Quality Management to Compete with the West - BioProcess InternationalBioProcess International

Frontiers | Advanced Therapy Medicinal Products' Translation in Europe: A Developers' Perspective | Medicine

Early Development GMPs for Drug-Product Manufacturing of Small Molecules: An Industry Perspective (Part III)













![The Quality System in the pharmaceutical context [Grandi Strumentazioni e Core Facilities (FAST)] The Quality System in the pharmaceutical context [Grandi Strumentazioni e Core Facilities (FAST)]](https://corefacilities.iss.it/dw/lib/exe/fetch.php?media=en:aree:fabiocell:sq.png)