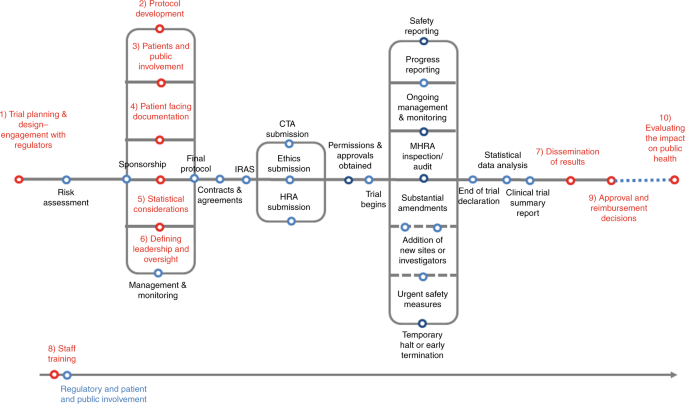
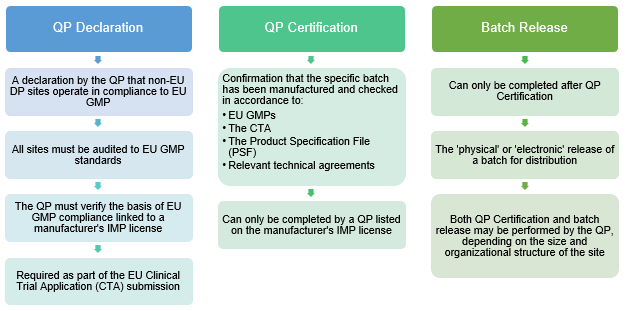
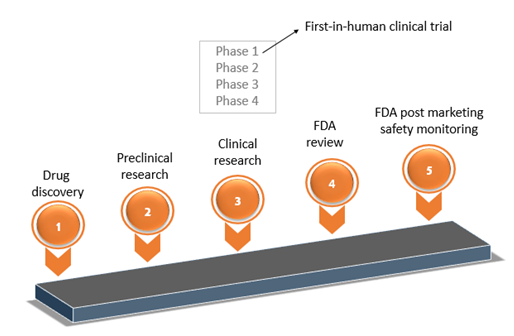
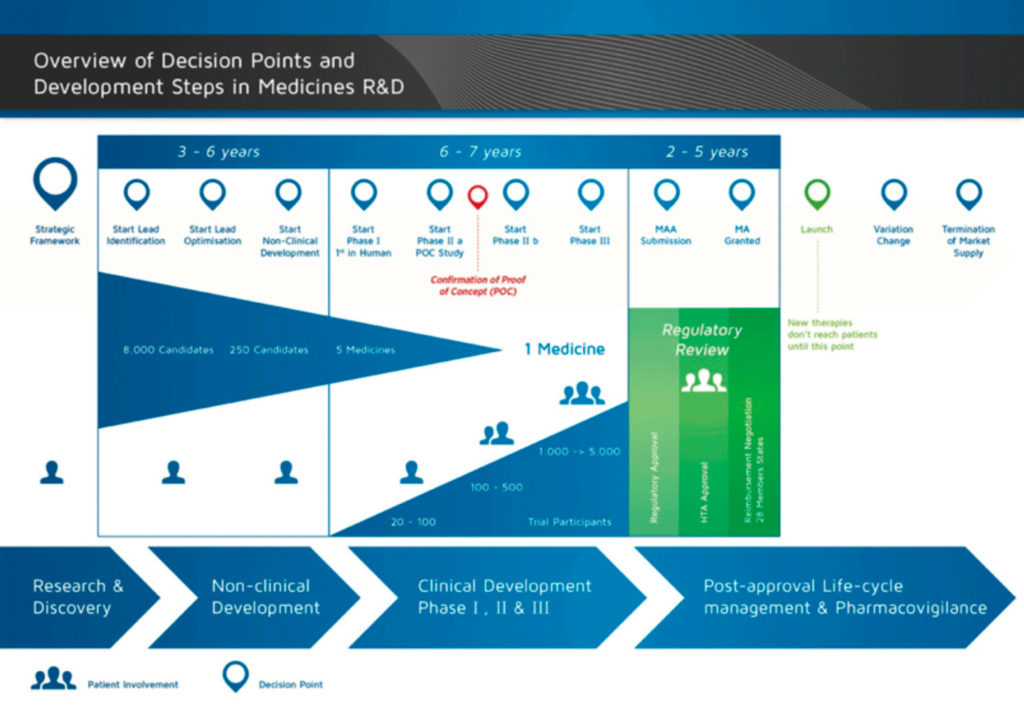
EORTC EU Clinical Trials Directives Organisation and Implementation of Cancer Clinical Trials Anastassia Negrouk EORTC Regulatory Affairs Manager Intergroup. - ppt download
The Clinical Trials Directive: How Is It Affecting Europe's Noncommercial Research | PLOS Clinical Trials
Frontiers | Transitioning From Preclinical Evidence to Advanced Therapy Medicinal Product: A Spanish Experience | Cardiovascular Medicine

Definition of Investigational Medicinal Products (IMPs) Definition of Non Investigational Medicinal Products (NIMPs) - PDF Free Download