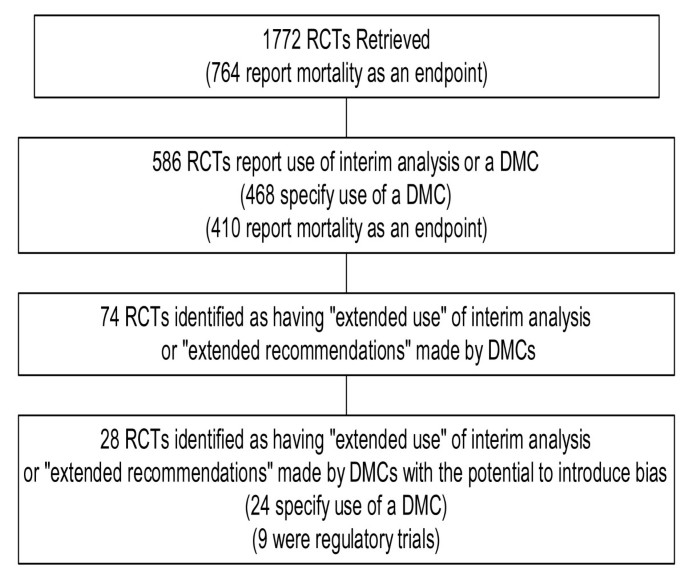
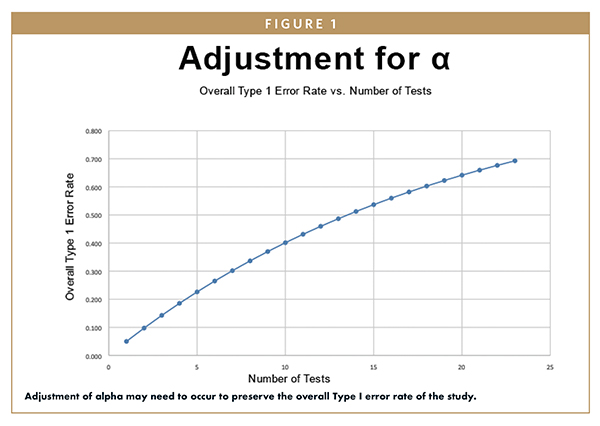
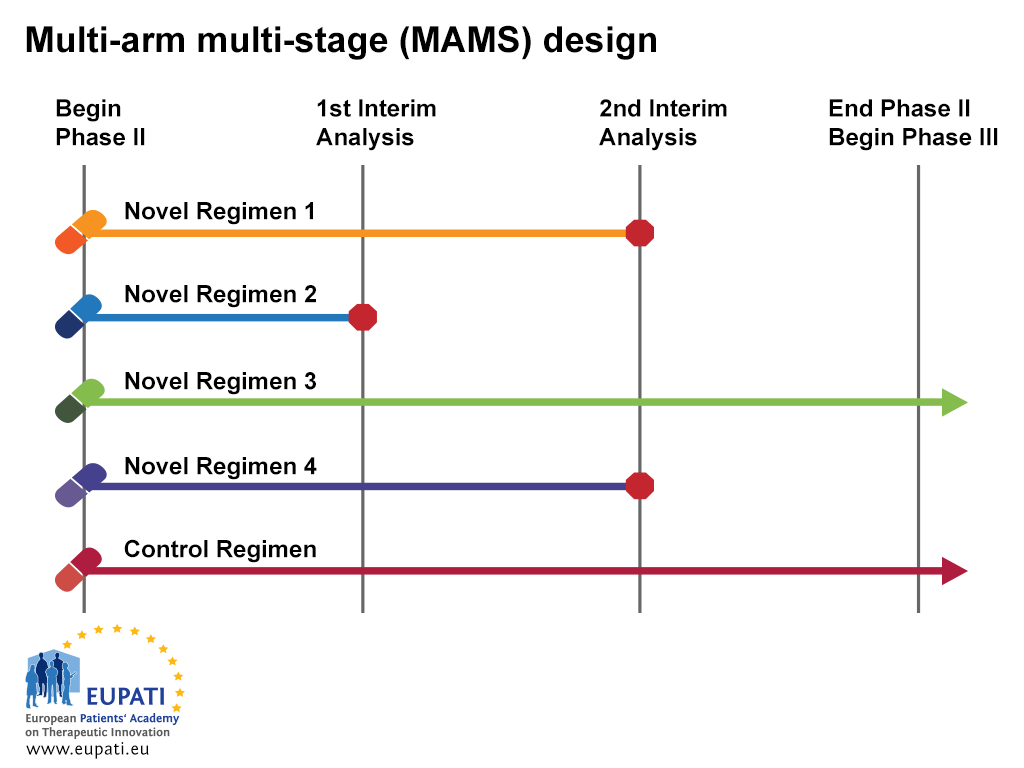
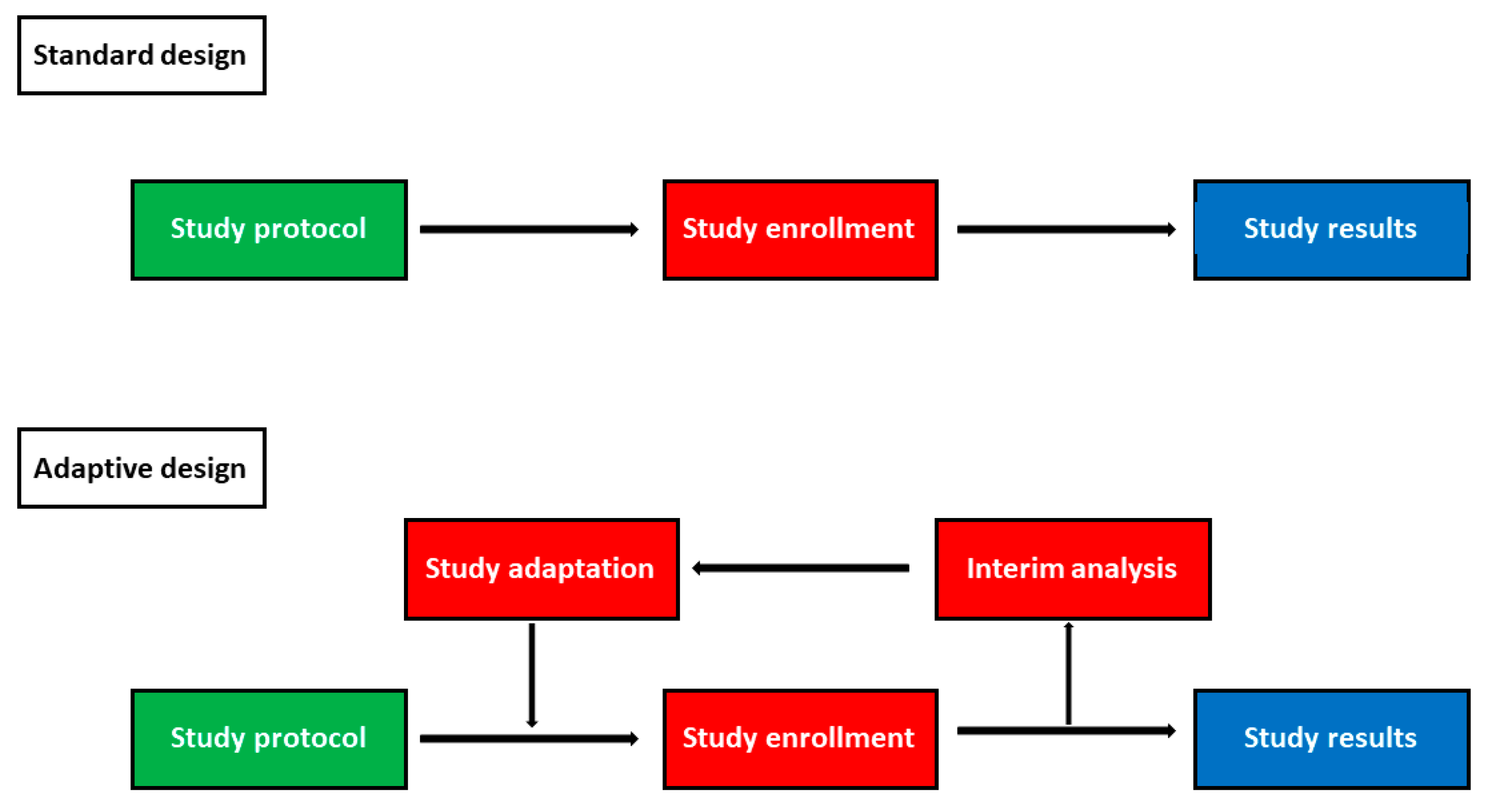
Clinical Trials - analysis End-points, planned and unplanned secondary analyses, interim analyses, stopping rules Dick Menzies, MD Respiratory Epidemiology. - ppt download

RenovoRx Presenting Phase III Clinical Trial Interim Analysis Data of the TIGeR-PaC Study at American Association for Cancer Research Annual Meeting Underway in Orlando, Florida - RenovoRx

On Biostatistics and Clinical Trials: VALOR Trial - A Successful and Failed Phase III Study with Adaptive Sample Size Re-stimation for Promising Zone

Sample size reassessment. Notes: If the first interim analysis shows... | Download Scientific Diagram
Evaluating Clinical Trial Designs for Investigational Treatments of Ebola Virus Disease | PLOS Medicine

Development and Evaluation of a Simulation-Based Algorithm to Optimize the Planning of Interim Analyses for Clinical Trials in ALS | Neurology
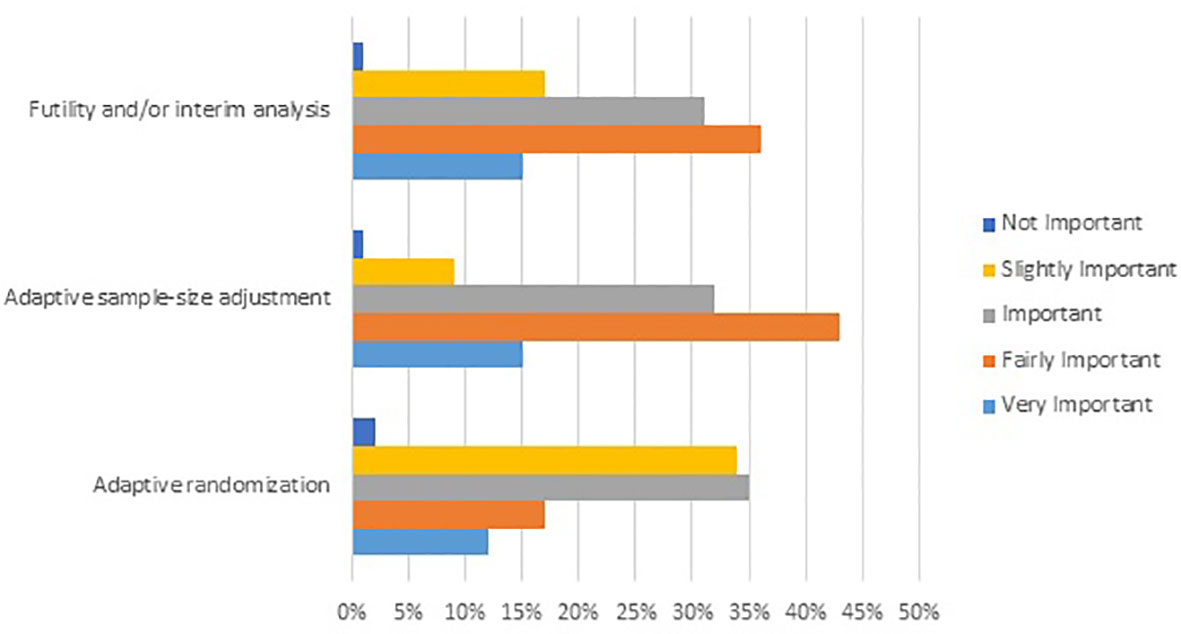
Frontiers | Value of Adaptive Trials and Surrogate Endpoints for Clinical Decision-Making in Rare Cancers