How Can a Systematic Literature Review (SLR) Help Prevent Common Pitfalls in Meeting the Elementary Requirements of Clinical Evaluation for MEDDEV 2.7 /1 revision 4 Clinical Evaluation Report (CER)? - Criterion Edge

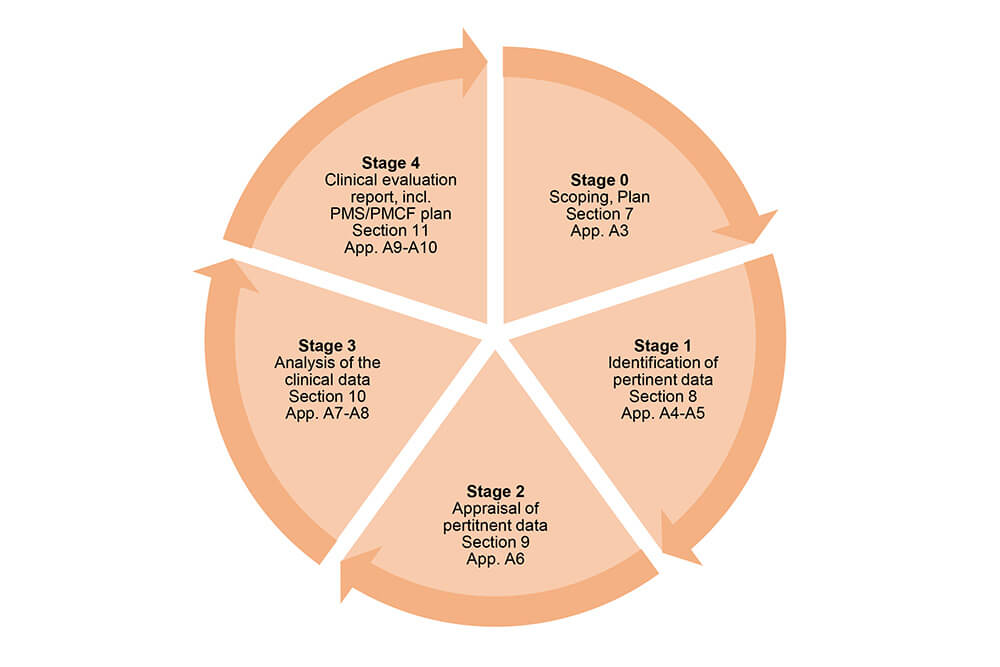
How to perform a clinical evaluation of medical devices – Part 3 – Suggested Table of Contents for the Clinical Evaluation Report – CER – Medical Device Expert News

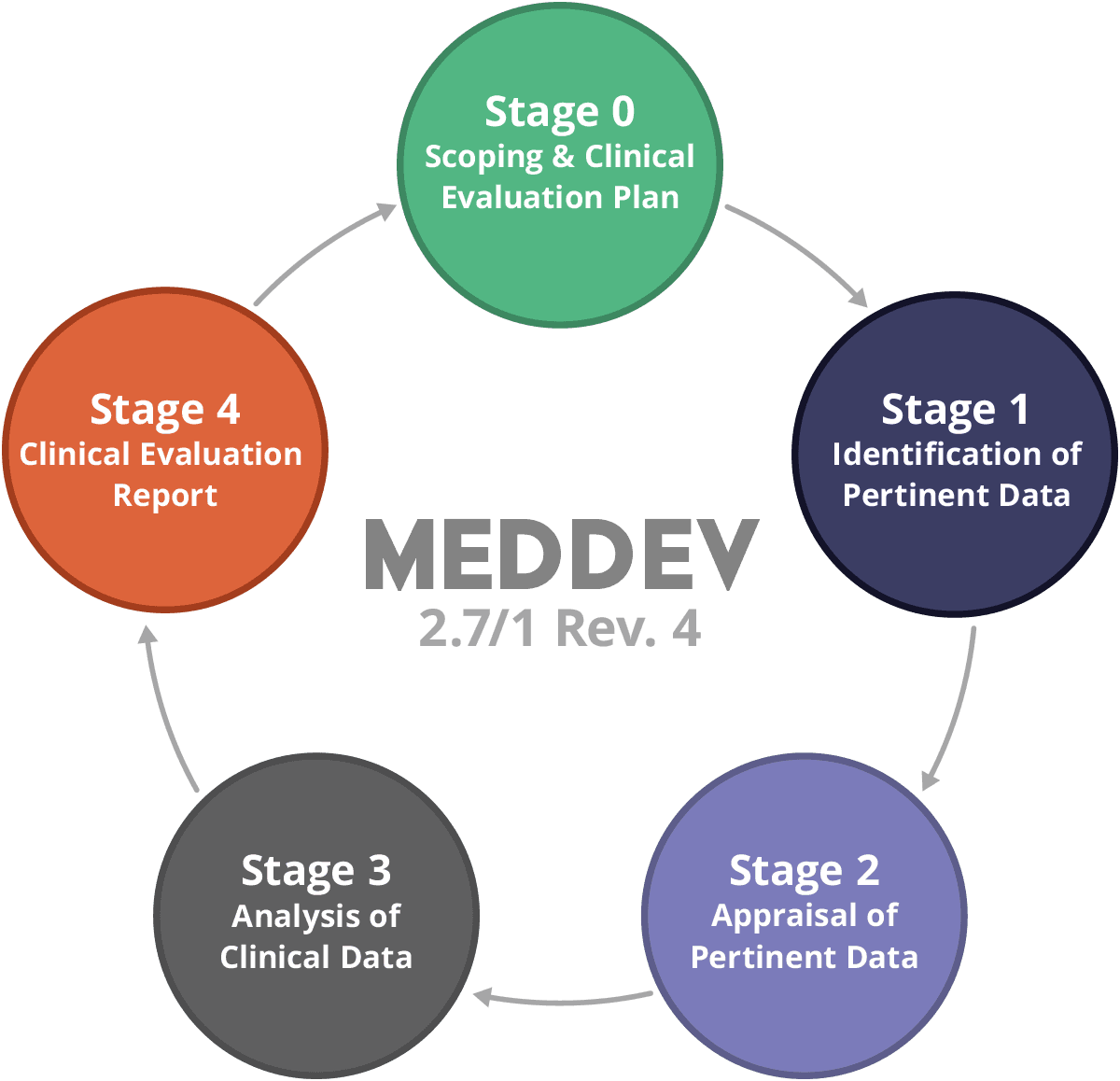
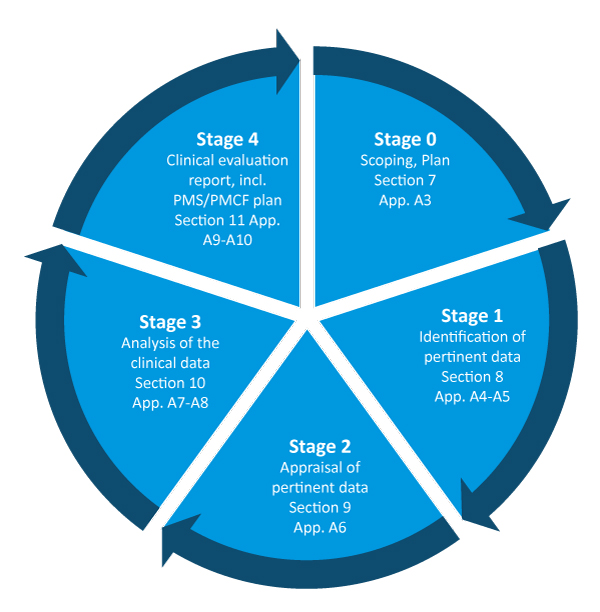
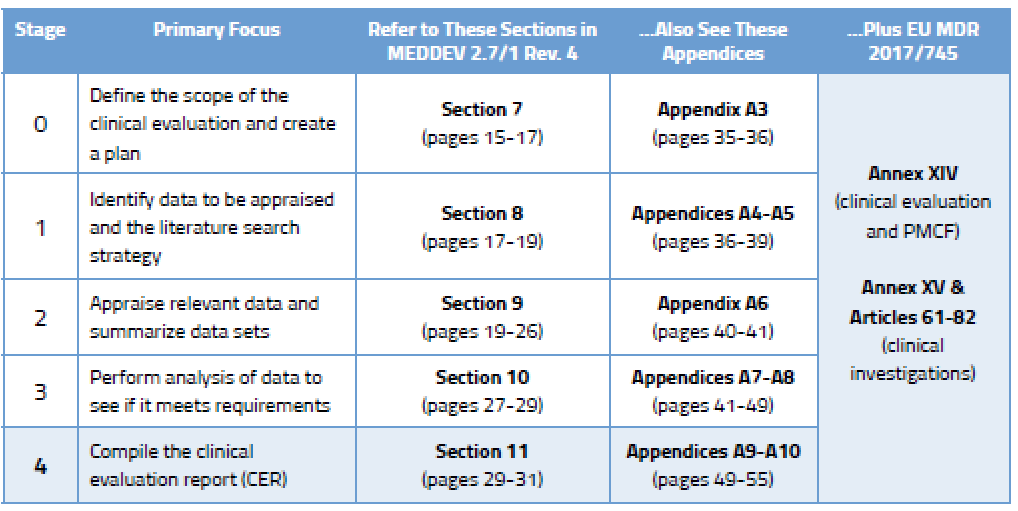
Medical devices #clinical evaluation report #MEDDEV 2.7/1 Rev. 4 (June 2016) #CER #TuracozHealthcareSolutions (#THS) – Turacoz Healthcare Solutions

A Clinical Evaluation Report (CER) that complies with MEDDEV 2.7.1. Rev 4 Guidelines: A Case of X-Ray and ECG – Guires News Room

Clinical evaluation: is your Clinical Evaluation Report (CER) compliant with MEDDEV 2.7/1 rev. 4? - Thema Med