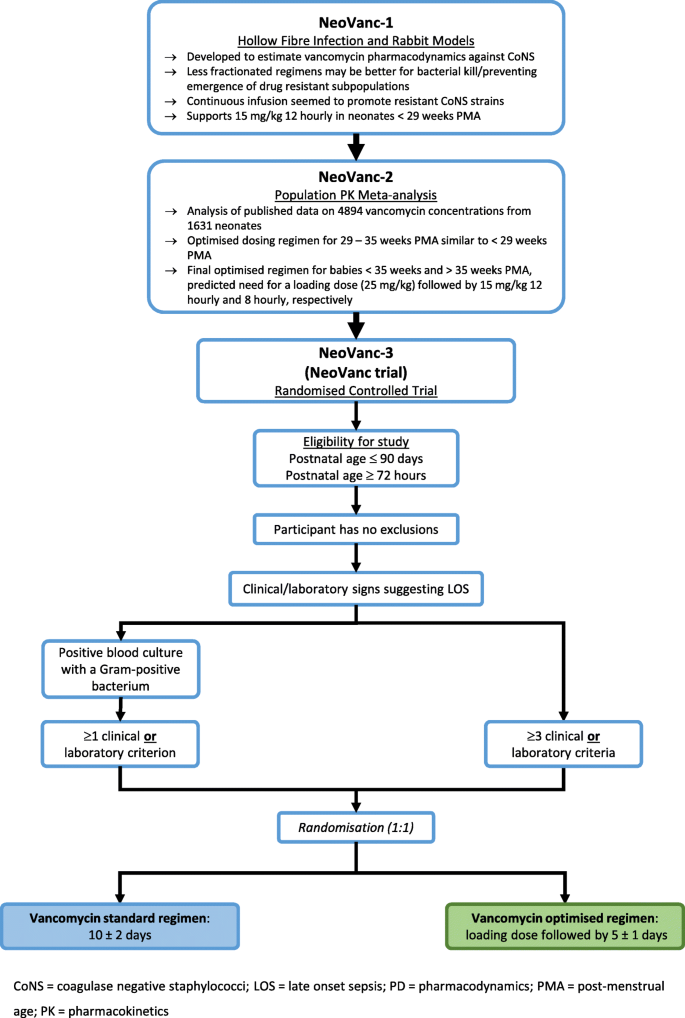
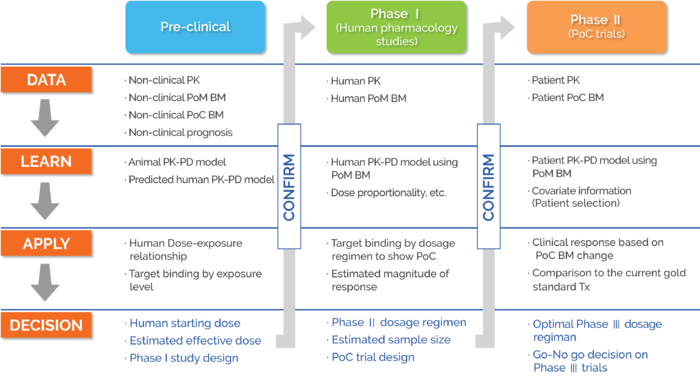
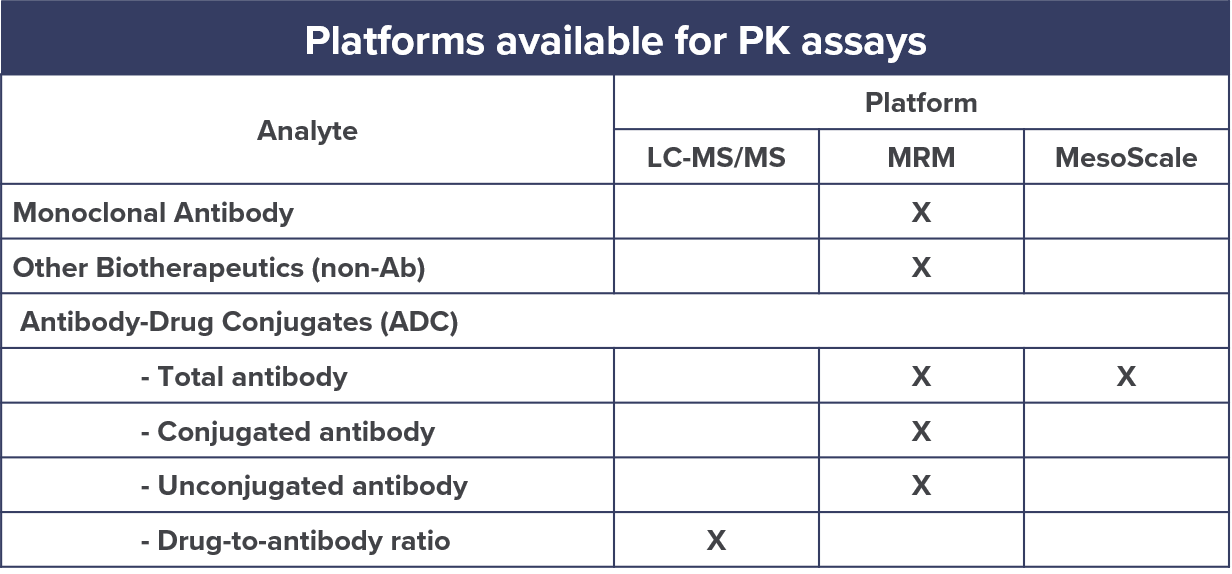
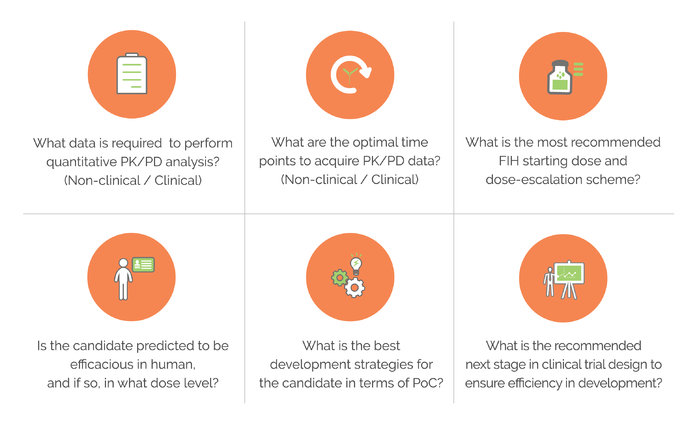
Clinical Pharmacology Regulatory Sciences in Drug Development and Precision Medicine: Current Status and Emerging Trends. - Abstract - Europe PMC
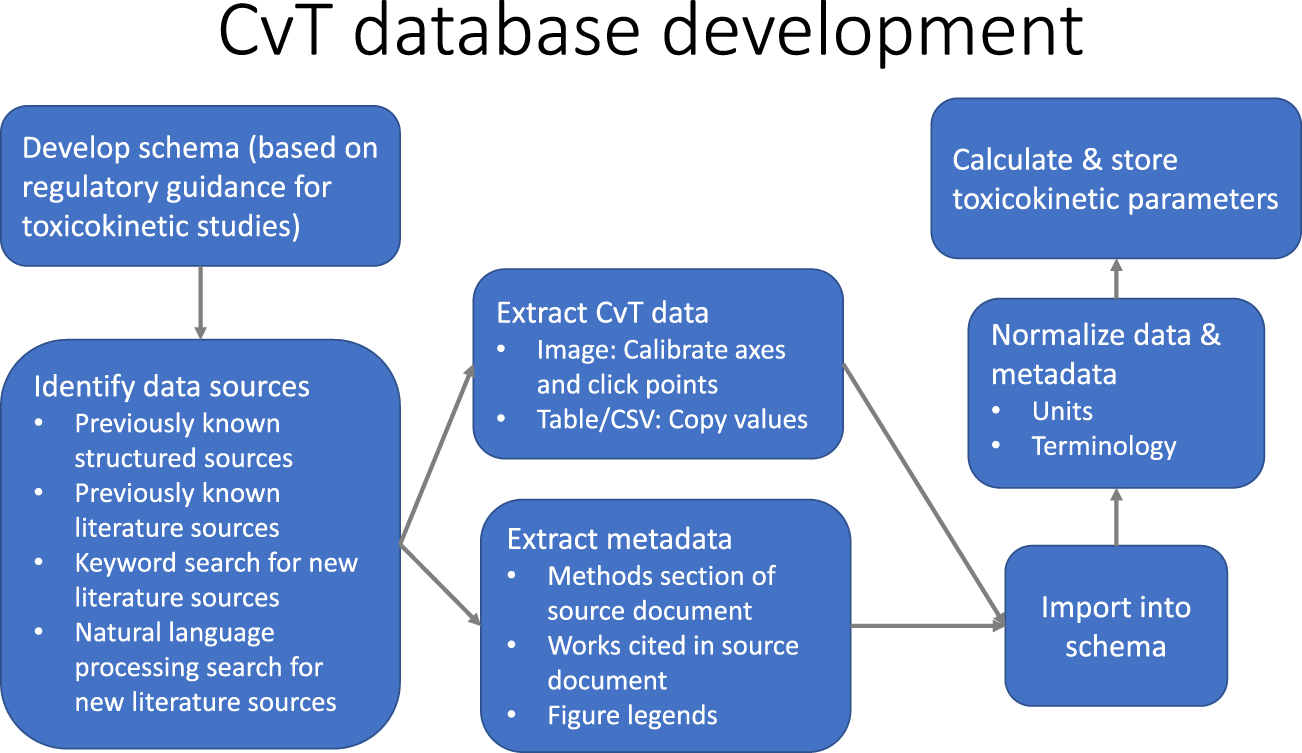
Database of pharmacokinetic time-series data and parameters for 144 environmental chemicals | Scientific Data

Correlating preclinical animal studies and human clinical trials of a multifunctional, polymeric nanoparticle | PNAS

Phase I, Open-Label, Dose-Escalation Study of the Safety, Pharmacokinetics, Pharmacodynamics, and Efficacy of GSK2879552 in Relapsed/Refractory SCLC - Journal of Thoracic Oncology

Pharmacokinetic and Statistical Considerations in First-in-Human Clinical Trials | Pharmaceutical Outsourcing - The Journal of Pharmaceutical & Biopharmaceutical Contract Services

Regulus Therapeutics Announces Positive Topline Safety and Pharmacokinetic ( PK) Data from the Phase 1 Single-Ascending Dose (SAD) Clinical Trial of RGLS8429 for the treatment of Autosomal Dominant Polycystic Kidney Disease (ADPKD) -

Recommendations for the Design of Clinical Drug–Drug Interaction Studies With Itraconazole Using a Mechanistic Physiologically‐Based Pharmacokinetic Model - Chen - 2019 - CPT: Pharmacometrics & Systems Pharmacology - Wiley Online Library
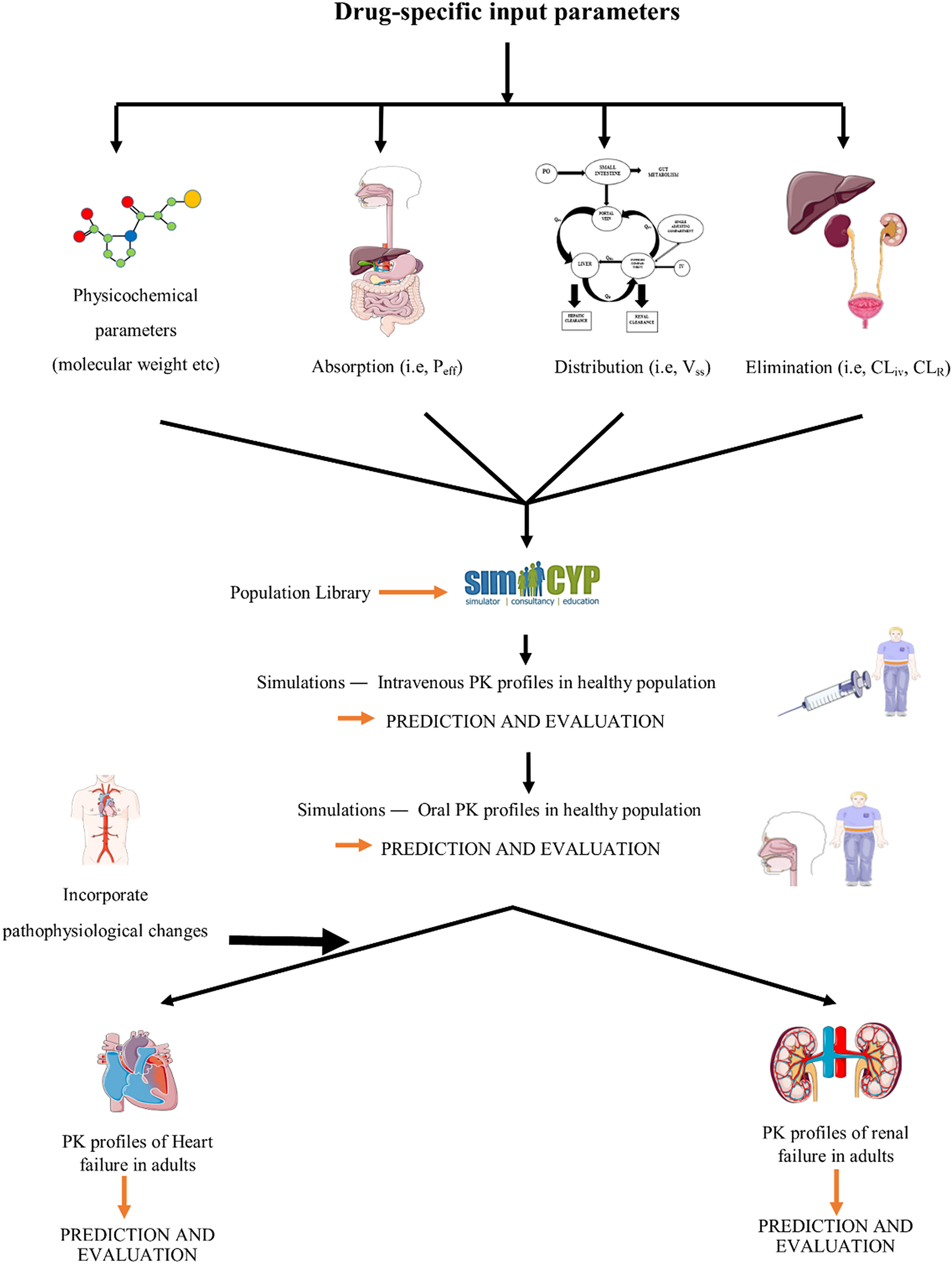
Development and evaluation of physiologically based pharmacokinetic drug-disease models for predicting captopril pharmacokinetics in chronic diseases | Scientific Reports

Use of old antibiotics now and in the future from a pharmacokinetic/pharmacodynamic perspective - Clinical Microbiology and Infection
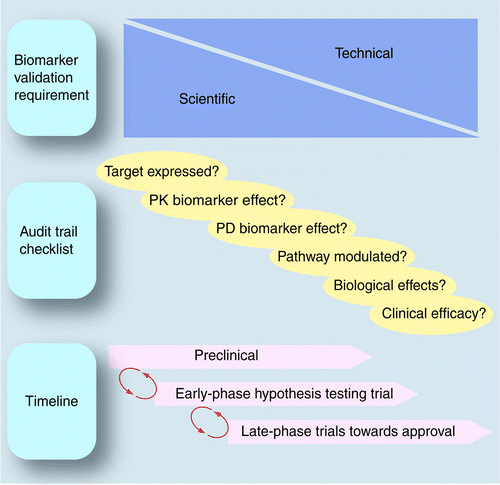
Use of pharmacokinetic/pharmacodynamic biomarkers to support rational cancer drug development | Biomarkers in Medicine